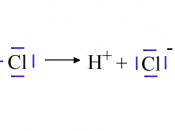Experiment to Investigate the Effect of Temperature on Rate of Reaction between Magnesium Ribbon and Hydrochloric Acid
Introduction
Magnesium (Mg) is a reactive metal, and therefore the solid is hold together by a metallic bond. A metallic bond is between metals only and they are all held together by a "sea of electron" which the electrons come from the metal atom. It has a mass number of 24 and a proton number of 12.
Hydrochloric acid (HCl) is an acidic compound it contains Hydrogen and Chlorine (Cl), Hydrogen (H) has a mass number of 1 and a proton number of 1 and Cl has a mass number of 35 and a proton number of 17. And they form a covalent bond between them. Covalent bond is between non-metals and the 2 elements share electrons with each other. Since Cl requires 1 extra electron in its outer shell to have a full outer shell it will only bond with one hydrogen atom.
When Mg comes into contact with HCl they react and form an ionic bond.
Magnesium + Hydrochloric acid "" Magnesium chloride + Hydrogen
Mg(s) + 2HCl(aq) "" 2MgCl(aq) +H2(g)
Aim
In this experiment I will be changing the rate of reaction by increasing the temperature of hydrochloric acid and drop a ribbon of Mg in the acid in different temperatures and to see how long it takes for the Mg ribbon to completely react with the acid.
Equipment
50cm" measuring cylinder
250ml beaker
Bunsen burner
Tripod
32cm of magnesium
200ml of hydrochloric acid
recording sheet
stop watch
thermometer
matches
Method
First I have to measure 50cm" of hydrochloric acid with a measuring cylinder, then I have to heat it up to the temperature that I want it to be, and I will measure the temperature with a thermometer, then...