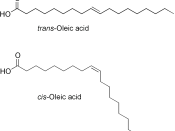What are isomers?
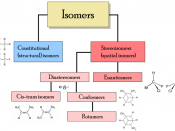
Isomers are molecules with the same molecular formula, but different arrangements of atoms. There are different types of isomers, they are
-------------------------
Structural Isomerism
------------------------
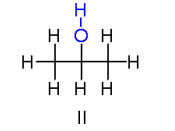
Structural isomerism occurs when two or more organic compounds have the same molecular formulae, but different structures. These differences tend to give the molecules different chemical and physical properties. There are three types of structural isomerism that you need to be aware of at school: chain isomerism, positional isomerism and functional isomerism. There is a fourth type, known as tautomerism (where there are two isomers are known as the keto and enol isomers) but you won't come across this at school.
---------------------
Stereo-isomerism
-------------------
Stereo-isomerism occurs when the atoms in a molecule can have different arrangements in space. There are two types of stereo-isomerism: geometrical isomerism and optical isomerism. Geometrical isomers can have very different physical properties, such as different melting points, but they tend to have the same chemical properties.
Optical isomers have the same chemical and physical properties, except that one structure rotates the plane of polarised light to the right and the other rotates it to the left.
-----------------------
Geometrical isomers
---------------------
Geometric isomers have the same structural formulas but differ in the arrangement of groups at a single atom, at double bonds, or in rings. Cis- and trans-platin (see Figure 37) are examples of geometric isomers based on the different arrangement of groups at a single atom. Cis- and trans-2-butene differ in the arrangement of the methyl groups about the double bonds.
Although geometric isomers have completely different physical and chemical properties (for example, cis- and trans-2-butene have different boiling points and densities), optical isomers (also called enantiomers) differ in only one characteristic--their interaction with plane polarized light. When a beam of light is passed through a certain...