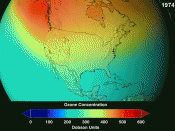
ÃÂThe ozone is a gas that occurs both in the EarthÃÂs upper atmosphere and at ground level; it can be ÃÂgoodÃÂ or ÃÂbadÃÂ for our health and the environment, depending on its location in the atmosphereÃÂ (ÃÂOzone- Good up high bad nearby ÃÂ par.1). The troposphere is the layer of ozone that starts at ground level and extends upward about 6-10 miles. This is the area where pollution and smog is located. ÃÂTropospheric ozone can permanently damage peopleÃÂs lungs and prohibit plants from producing and storing foodÃÂ (Baker, par. 2). The troposphere is referred to as the ÃÂbadÃÂ ozone. The next layer of ozone is the stratosphere, which stretches up 6-30 miles. The stratosphere is considered ÃÂgoodÃÂ because it shields the Earth from harmful effects of ultraviolet rays from the sun by absorbing most of their waves.
It starts to get ugly in the stratosphere, thanks to certain human inventions.
ÃÂÃÂ this ÃÂgoodÃÂ ozone is gradually being destroyed by man-made chemicals referred to as ozone-depleting substances (ODS), including chlorofluorocarbons (CFCs), hydrochlorofluorocarbons (HCFCs), halons, methyl bromide, carbon tetrachloride, and methyl chloroform. These substances were formerly used and sometimes still are used in coolants, foaming agents, fire extinguishers, solvents, pesticides, and aerosol propellantsÃÂ (ÃÂOzone- Good up high bad nearbyÃÂ par. 3). It takes decades and even centuries for these chemicals to degrade. These substances have essentially destroyed a part of the stratosphere because they release chlorine and bromine molecules during their breakdown. The chlorine and bromine molecules attack ozone molecules.
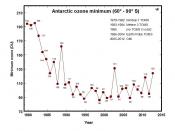
The greatest amount of reduction in the stratospheric ozone is in the Antarctic, during the Antarctic spring. This is what we refer to as the ozone ÃÂhole.ÃÂ There is also a less pronounced area of ozone depletion in the Arctic during the Arctic spring. It is cold enough in the polar...