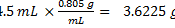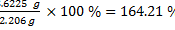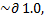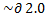Nor Amirah Farhana Nawawi, Mia Organic Chemistry Lab Report 2 Jessica Sammons
Title: Preparation of 2-butanone
Introduction: The goal of this experiment was to prepare 2-butanone from 2-butanol. Chromic acid was used in this experiment to in order to prepare 2-butanol. Cr (VI) is rather orange, but Cr (III) is dark green - therefore by oxidizing the alcohol (2-butanol), an orange Cr (IV) is reduced to green. NMR and IR tests were taken to determine the result, and the crystallized derivative of this product was obtained.
Procedure: The experiment followed the instructions in the lab manual, except for the empty heating mantle, which our TA advised us to use with sand instead of heating it empty.
Results and calculations:
Weight of crude product
Density of 2-butanone: 0.805 g/mL
Volume of crude product: 4.5 mL
Mass of crude product:
% yield product
% theoretical yield: 2.206 g
% actual yield: 3.6225
g
�
Weight after simple distillation
Volume of product: 2.2 mL
Mass of product: 1.771 g
Percent yield of redistilled product
Melting point of derivative: 230ÃÂC -236ÃÂC
Conclusion
In this experiment, 2-butanone was prepared from 2-butanol, oxidized by chromic acid. The percent yield of the product is 164.21 %, which is more than the theoretical yield. This happened because steam was distilled along with the 2-butanone at higher temperature. However, this was not a mess, because the product was redistilled with simple distillation, and the correct compound was obtained later by achieving an 80.28 % of redistilled product. Getting a 100 % yield might be impossible, but this is the closest to perfection, because there are always some product lost in the air while distilling, and some in the Erlenmeyer flask with the drying magnesium sulfate.
The derivative of this product was obtained by adding the 2-butanone, freshly made, with 2,4-dinitrophenylhydrazine solution (DNP) to create 2,4-dinitrophenylhydrazone. This compound is solid, and stable as it is incompatible with strong oxidizing reagent.
�
Questions
Q1)
IR analysis
When analyzing the IR spectra, these are few that were observed. There is a peak around 1700 cm-1, indicating the carbonyl group, C = O bond (1600 - 1800 cm-1). This proved that the product had turned to carbonyl on stead of the alcohol, O - H bond that it used to. The O - H bond, which would be very easy to recognize in an IR because of its broad stretch around 3200 - 3500 cm-1, is no longer there. The product is therefore almost pure without unreacted reactant.
NMR analysis
In a 2-butanone, there are three protons at C-1, none at C-2 (the C=O bond is here), two at C-3, and three at C-4. All the protons / hydrogen give different signals, or have different chemical shifts, because there is no symmetry in this molecule. Referring to the attachment of the H NMR result, there are four significant peaks - ignore the first on the left because it has nothing to do with the compound, and so now we are left with three peaks. The first peak, (we'll start from the right) with chemical shift reading around
The second peak from the right shows a singlet with three protons, with chemical shift
Derivative
The derivative was made because 2-butanone is present in liquid form under room temperature. So, 2,4-dinitrophenylhydrazone was made to determine that product earlier was really 2-butanone. The actual melting point of 2,4-dinitrophenylhydrazone is 239ÃÂC - 241ÃÂC, and the experimental melting point was 230ÃÂC - 236ÃÂC, which is very close to the actual. This shows that the reaction really makes 2-butanone.
Q2)
The oxidation with chromate is not general for all alcohols, as only secondary and primary alcohol would be oxidized this way - not a tertiary alcohol.
Q3)
If 1-butanol were oxidized, the product that would be produced is butanoic acid; a carboxylic acid.