INTRODUCTION
The aim of this assignment is to examine the production of a pharmaceutical intermediate. A method of processing is to be selected and justified, and a scale of the operation identified including the major items of processing plant and method operation. The implication of the selection with respect to safety, health and environment should be outlined for current and future operations.
The reaction was initially carried out in chloroform. Due to the undesirable health properties of chloroform it was substituted by dichloromethane. Reaction times with this solvent were extended and it was subsequently replaced by an excess of Liquid A.
The dissolution of Solid A in Liquid A and the subsequent reaction both appear to be endothermic. Gaseous waste products from the reaction can be a problem if not controlled or abated. Liquid A loss may also be significant. Neither of the reactions will proceed satisfactorily at room temperature.
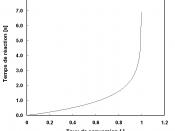
The reaction of the Solid A and Liquid A is much faster than the break down of the intermediate to Liquid B. In order to raise the reaction rate of the second reaction the temperature must be raised and to achieve this the excess Liquid A is removed by distillation. Typical reaction/temperature curves are appended. The typical reaction efficiency is 97% with respect to Solid A.
Final recovery of the Liquid B is by distillation at 55 deg. C under vacuum.
There are some solid wastes from the reaction including the residual Solid A. These wastes are soluble in Liquid B.
The required purity of Liquid B is >99%.
Initial production rate is: 100 T/a
The forecast demand is: 110 T/a in year 2
125 T/a in year 3
160 T/a in year 4
206 T/a in year 5
There is no forecast beyond year 5 but it is anticipated...


Very indepth
Alot of indepth deatils and facts, well done
1 out of 3 people found this comment useful.