Structure and two functions of proteins
Introduction
Proteins are divided into two groups, animal proteins and plant proteins they are polymers and are found in every living thing with its shape defining its function. The protein shape is categorised as either globulous protein or fibrous protein. They consist of chains of small molecules called amino acid which determine the protein structure and therefore its function. Proteins can be either Fibrous which is when the protein consists of long insoluble polypeptide chains or Globular which describes a round protein containing a number of polypeptide chains. They serve a significant purpose and are very complex. Proteins do a majority of the cell work and regulate the body's tissues and organs. Here the protein structure is discussed and the four levels of organisation examined. Proteins have a number of functions, in this essay two of those functions are looked at and there purpose explained.
The structure of a protein consists of four levels of organisation which are distinguished from one another by the degree of complexity within the polypeptide chain.
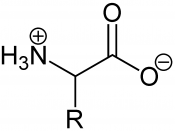
The first of which being the Primary structure which consists of a sequence of amino acid bonded together to form a polypepticle or a chain of amino acid. The linear sequence of twenty amino acids complete the numerous proteins within the body, each amino acid having different side chains. The side chains are the part of the amino acid that defines it from another amino acid. All amino acids contain an amine group, a carboxyl group, as well as the individual chain. The sequence that the amino acid take determine which bonds form and how the protein folds into its structure. . How they connect is dependent on the genetic code that comes from the DNA.
Figure 1, peptide chain (hanguyenbiologyhlblog, 2013)
Once a primary structure is formed a secondary structure begins to evolve. The secondary structure occurs when the long polypeptide chain folds. It is held together by hydrogen bonds of the polymer, these bonds create Alpha helix (a) chained or Beta (b) sheets. The shape on which the polypeptide chain takes is a result of ionic bonding that occurs between metals , non-metals and hydrogen bonding.
Figure 2 (hanguyenbiologyhlblog,2013)
Tertiary structure is decided when the regions of the secondary structure are folded together in a specific manner. It is a delicate structure and easily altered. The bending and twisting of the polypeptide form a precise globular shape. The tertiary structure is affected by varied bonds which include Ionic interactions, disulfide bonds, hydrophobic -hydrophilic interactions and hydrogen bonds.
Quaternary structure
For most protein structures the organisation stops after the tertiary structure, others contain the quaternary structure. This occurs when the protein has more than one string of amino acid, and when these connect it is called the quaternary structure. Proteins consisting of more than one chain of amino acids are called oligomeric proteins. This forms a large complex protein molecule.
Figure 4 (biochemistryquestions, 2008)-
Each Protein has a specific function with some involved in supporting either structural support, bodily movement or work in helping defend against germs. Here two of those functions are examined.
Antibodies are specialised proteins known as immunoglobulins, and they are produced by lymphocytes from the immune system in which they play an important role. They help in preventing infection, with many vaccines relying on an antibody reaction to work properly as they bind to bacteria or viruses. They consist of two short light polypeptide chains and two heavy long polypeptide chains. The antibody shapes resembles that of a "Y" (See figure 4). Antibodies are made from white blood cells and will typically only recognise eight to ten amino acid. If the sequence of carbon and nitrogen molecules was disturbed the antibodies would no longer function. They can be used to detect even small particles of protein on the cell surface.
Figure 5
Enzymes
Enzymes are large molecules found in organisms they control the activity within the cell and are three dimensional in structure. They facilitate biochemical reactions and have often been referred to as catalysts as they increase the speed of the chemical reactions. All bodily functions include enzymes at a majority of their operational stages and they are a necessity for the body to heal itself and resist disease. All enzymes only function with specific substrates. They are biological catalysts that have optimum temperatures and pH values. When the temperature raises so does the rate of reaction although temperatures above 45â° and the enzyme becomes denatured. The area that fits the substrate molecules is called the active site see figure 6, enzymes contain an active site (mostly only one) which is a small port. This port facilitates the substrate molecules that bind producing a chemical reaction, see figure 6.Specific enzymes contribute to almost all activity that happens at the cellular level within the body.
Figure 6
References
Berg,JM, Tymoczko, JL, Stryer L. (2002) . Chapter 3 Protein Structure and
Function. Available: http://www.ncbi,nlm.nih.gov/books/NBK21177.
Bowen,R.(2002). The Structure of Proteins: Available :
http://www.vivo.colostrate.edu/hbook/genetics/biotech/basics/prostruct.htm.
CGP Books,2008. As - Level Biology Complete Revision & Practice. Revised
Edition. Coordination Group Publications Ltd CCGP
Dawn, A. (2011). What are Antibodies.
Available:http://www.faculty.stcc.edu/AandP/AP/APZpages.Units21to33/immune/abinfo.htm