Radioactivity
By: lilticklemeelmo_23
Radioactivity a.k.a. radioactive decay, is the steps taken in which the atomic nuclei of unstable isotopes release fast-moving particles and energy. There are three types of radioactive decay: (each based on which kind of radiation released by the nucleus) alpha decay, beta decay, and gamma decay.
Gamma rays, also called gamma radiation, are the most energetic radiation. In a radioactive isotope, the gamma rays come from the nucleus of the atom. There are two ways radiation can travel either as particles or waves. It does not change the atomic mass or the atomic number of the atom that was formed. If it travels through a human body, it will cause damage to cells. These rays are very penetrating. They are similar to x-rays ( they can travel through about anything thick or thin like concrete, paper, and aluminum).
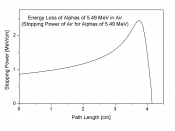
Alpha decay (alpha particle) contains two protons and two neutrons.
These particles can move very fast. It looses energy quickly. Alpha radiation can cause a sore much like a bad burn.
Beta decay (beta particle) is an electron given off by a nucleus during radioactive decay. There are two types of beta decay such as beta-minus and beta-plus. During beta-minus decay, a neutron in an atom's nucleus turns into a proton. During beta-plus decay, a proton in an atom's nucleus turns into a neutron. Even though the numbers of protons and neutrons in an atoms nucleus vary, the total of particles stay the same.
Half life describes the amount of time needed for half of a sample of unstable atoms or particles to undergo decay.
Isotopes are atoms with the same number of protons and different numbers of neutrons. Isotopes are used in modern medicine for research and diagnosing diseases. Example: carbon-14 has a mass number of...