Radon: Our Major Source of Radiation Dose
Introduction
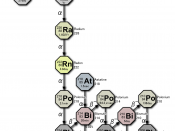
Radon-222 is a natural, radioactive isotope of element number 86, that
occurs in the uranium-238 decay chain (see table 1). Its immediate parent is
radium-226, and radon-222 itself decays by alpha particle emission through a
series of short -lived decay products (mainly isotopes of polonium, lead and
bismuth) to lead-210 and on eventually to stable lead-206.
Element Radiation Half-Life
Uranium | 238 alpha 4,460,000,000 years |
Thorium | 234 beta 24.1 days |
Protactinium | 234 beta 1.17 minutes |
Uranium | 234 alpha 247,000 years |
Thorium | 230 alpha 80,000 years |
Radium | 226 alpha 1,602 years |
Radon | 222 alpha 3.82 days |
Polonium | 218 alpha 3.05 minutes |
Lead | 214 beta 27 minutes |
Bismuth | 214 beta 19.7 minutes |
Polonium | 214 alpha 1 microsecond |
Lead | 210 beta 22.3 years |
Bismuth | 210 beta 5.01 days |
Polonium | 210 alpha 138.4 days |
Lead | 206 none stable |
Table 1. Uranium | 238 Decay Chain |
Two other isotopes of radon occur in nature; radon-220, which occurs in the
thorium-232 decay chain (table 2) and radon-219 in the uranium-235 chain
(table 3).
Both these isotopes have half-lives of under a minute and are less
important than radon-222 which is the subject of the rest of this essay.
Element Radiation Half-Life
Th | 232 alpha 14,000,000,000 years |
Ra | 228 beta 5.76 years |
Ac | 228 beta 6.13 hours |
Th | 228 alpha 1.9 years |
Ra | 224 alpha 3.66 days |
Rn | 220 alpha 55.6 seconds |
Po | 216 alpha 0.145 seconds |
Pb | 212 beta 10.6 hours |
Bi | 212 beta 61 minutes |
Po | 212 alpha 0.3 microseconds |
Tl | 208 beta 3 minutes |
Pb | 208 stable 22.3 years |
Table 2. Thorium-232 Decay Chain
U | 235 Alpha 704,000,000 years |
Th | 231 Beta 1 day |
Pa | 231 Alpha 32,500 years |
Ac | 227 Alpha 21.8 years |
Fr | 223 Beta 21.8 minutes |
Ra | 223 Alpha 11.4 days |
Rn | 219 Alpha 4 seconds |
Po | 215 Alpha 1.78 milliseconds |
Pb | 211 Beta 36 minutes |
Bi | 211 Alpha 2.15 minutes |
Tl | 207 Beta 4.77 minutes |
Pb | 207 Stable |
Table 3. Uranium-235 Decay Chain
Radon is one of the group 0 elements, the noble gases, and is, therefore,
chemically virtually inert. However, it has been reported that fluorine
reacts with radon, forming a fluoride.
On average, one part of radon is present in 1 x 1021 parts of air. At room
temperature radon is a colourless gas; when cooled below its freezing
point (-71 C), radon exhibits a brilliant phosphorescence which becomes
yellow as the temperature is lowered and orange-red at the temperature
of liquid air. Liquid radon boils at -61.7 C.
It is formed in the environment by the decay of the trace amounts of
naturally occurring radium present in all rocks and soils. With a half-life of
3.8 days, it can migrate considerable distances through the ground, and
escape into the air. Outside, radon quickly disperses and levels are low -
about 4 Bq m-3, but indoors levels are higher - about 20 Bq m-3 on
The radioactivity of radon is measured in Becquerels per cubic metre of air
(Bq m-3). The Becquerel is named after Henri Becquerel (see essay number
1). A Becquerel is one radioactive disintegration per second.
average and can reach over 1,000 Bq m-3. When we breathe in air containing
radon, and its short-lived decay products, they irradiate our lungs. When
radon levels are high this causes a significant increase in the risk of lung
cancer.
This essay explains the concern about radon, explores the evidence that it
causes harm to humans, and provides details of the concerted effort by the
authorities in the UK to tackle the existing situation, and to reduce the risk in
the future. But first a little bit of radon history.
History of Radon
Friedrich Dorn, a German scientist, discovered in 1900 that radium was giving
off a gas which he called radium emanation. A few years later, in 1908,
William Ramsey and R.W. Whytlaw-Gray isolated enough of the gas to study
its physical properties. As well as finding it was the densest gas known (9.73
g dm-3), they called it niton. In the 1920's, the name radon, symbol Rn, was
adopted for all the isotopes of element 86.
Friedrich Dorn William Ramsey
Though radon was not discovered until 1900, the effects of prolonged
exposure to high levels had been noted over 300 years earlier. Two
researchers in the first half of the sixteenth century, Georgius Agricola (a
German physician and geologist, 1494 to 1555) and Paracelsus (a Swiss
physician, alchemist and scientist, 1493 to 1541) studied the diseases of
underground miners in Europe. They found that many miners died early
because of lung diseases, and concluded that the causes were dust and gases
in the mines. Studies in more recent times have shown that high radon levels
in mines in many parts of the world are linked to a higher risk of lung cancer.
Agricola Paracelsus
Concern about Radon
Everybody is exposed to radiation from a variety of sources (see essay
number 6 for more details). In the UK, 50% of the annual dose to the
average person comes from inhalation of radon and its short-lived decay
products, so radon is the major source of radiation exposure for almost all the
population. In addition, a significant number of people, perhaps a quarter of a
million or so, receive annual doses of 10 mSv.
Annual dose of radiation in UK (NRPB)
It is well known from studies of the survivors of the atomic bombs dropped on
Japan, some early medical procedures, and events such as the Chernobyl
accident that radiation can cause cancers. Using the data from these studies,
it is possible to estimate risk factors for the much smaller environmental
radiation exposures we all receive. However, these risk factors are so small it
is normally not possible to observe them directly for each source of exposure
against the natural background cancer rate. However, the exception is radon
as the exposures are often more bigger for some sections of the population.
The Uses of Radon
Radon is still produced for therapeutic use by a few hospitals by pumping it
from a radium source and sealing it in minute tubes, called seeds or needles,
for application to patient. This practice has been largely discontinued as
hospitals can get the seeds directly from suppliers, who make up the seeds
with the desired activity for the day of use.
There are still places where bathing in radon laden water is thought to be
healthy for the body and soul. One such place is the Rudolf-Stollen mine that
uses radon inhalation as a healing tool.
A radon monitoring method is employed in the Chuko fault zone in south
central Taiwan for earthquake prediction. Soil gas radon is monitored
continuously with a solid-state detector and recorded with a data logger. The
detector assembly is housed in a PVC pipe to reduce the influence of
environmental factors. The fault zone is known to have deep source gases
and sensitive to earthquake activities. The quantities of natural gas releases
are known to vary with earthquake activities. Data retrieval from the end of
October 2000 to the end of February 2001, showed that spike-like radon
anomalies, i.e. rapid increases in the amount of radon, occurred before every
major earthquake with a magnitude of more than 4.0 on the Richter scale.
The strong correlation between spike-like anomalies and major earthquakes
suggests that this might become a method of earthquake prediction.
The Evidence for the Risk from Radon
The first populations to be studied in detail were groups of underground
miners. Some 60,000 miners were involved in 12 main epidemiological
studies. The miners worked underground for significant periods between the
years 1941 and 1990 in 12 different groups of mines in 8 different countries.
Many of the mines produced uranium ore, but others were iron, tin and
fluorspar mines. Over 2,600 lung cancers were observed in these miners,
which is far more than the 750 predicted on the basis of the number of
cancers in the appropriate general population.
Subsequently, studies have been carried out to look for a direct link between
radon in the home and lung cancer. Two of the biggest were in Sweden and in
the Southwest of England. The study in Devon and Cornwall involved 982
individuals with lung cancer and 3,185 matched controls. A number of
analyses were carried out and most indicated that higher lung rates were
found in those exposed to higher levels of radon. However, in most cases the
result did not reach statistical significance so the conclusion was that the
overall result was compatible with the results of the analyses of the study of
the miners. This indicates that 5% of all lung cancers in the UK are caused by
radon.
Another study was performed in Gansu province, China, of people who move
house very rarely and suffer high mean radon levels. Also many dwellings
are below ground. Measurements were made with two one-year alpha track
detectors. The mean radon concentrations were 230 Bq m-3. These are
approximately ten times higher than average UK values.
While the general picture was of a clear association between domestic radon
exposure and lung cancer, one observation was striking. The association
seemed stronger in those living in below ground dwellings (439 cases), rather
than above ground houses and apartments (329 cases). The study concluded
'that effects of residential radon may equal or exceed miner-based estimates,
which are currently used to evaluate risk'.
The Mechanism for Harm from Radon
Radon is present in air in very small concentrations and it moves in and out of
our lungs with the rest of the air in the normal process of breathing. When
radon in the air decays into its daughter products, it forms atoms of solid
elements which are negatively charged. These anions attach themselves to
the small dust particles in the air to form a radioactive aerosol. When we
breathe these particles into our lungs, they stick to the lung-lining and are
not exhaled. As they are still radioactive, they irradiate the lung tissue. The
polonium-214 and -218 emit highly energetic alpha radiation causing damage
to the DNA of cells lining the lungs. Most of the damaged cells are killed.
However, some cells are partially damaged and get replicated. These cells can
induce lung cancer.
The Radon Programme in the UK
It was not until the latter half of the twentieth century that it was realised
that high radon levels in homes were a matter for concern. In the last thirty
of so years, much work has been done in the UK by the National Radiological
Protection Board (NRPB) with the full support of successive governments. A
strategy to control and reduce excessive exposure to radon has been
developed and maps, based on many measurements in homes, show the
areas with the greatest risk of high radon levels. Each local council will have
such a map for their area.
Radon Map of England and Wales from the NRPB
How to Measure Radon
There are many different methods of measuring radon levels. The two most
important are activated charcoal adsorption and alpha track detection.
For activated charcoal adsorption, an airtight container with activated
charcoal is opened in the area to be sampled, and radon in the air adsorbs
onto the charcoal granules. At the end of the sampling period, the container
is sealed and may be sent to a laboratory for analysis.
In alpha track detection, the detector is a small piece of special plastic or film
inside a small container. Air being tested diffuses through a filter covering a
hole in the container. When alpha particles from radon and its decay products
strike the detector, they cause damage tracks. At the end of the test the
container is sealed and returned to a laboratory for reading.
The detector is treated to enhance the damage tracks and then the tracks
over a predetermined area are counted using a microscope or optical reader.
The number of tracks per area counted is used to calculate the radon
concentration of the site tested.
Reducing Radon Levels in Existing Houses
One or more of the following methods may be used to reduce the radon level
in an existing building. A 'sump' and extract pipe can be installed beneath
the floor, from outside. A fan can be fitted to draw out the radon and blow it
into the atmosphere above the roof of the house. Gaps between the ground
floor and walls and gaps around service pipes can be sealed. Positive
pressurisation of the house using a fan in the roof-space prevents gas
entering, i.e. making the air pressure inside slightly higher than outside. Also
natural or forced ventilation of the void under the ground floor will reduce
radon levels.
How to Prevent High Levels in New Buildings
New buildings are generally protected by full protection to a suspended
concrete ground floor. In this the radon-proof barrier is positioned over the
floor structure and linked to cavity trays at the edges. Supplementary
protection is also provided by locating under floor vents on two or more sides
of the under floor space. If necessary the rate of ventilation and radon
dispersion can be increased by fitting an electric fan at a later date.
The radon barrier comprises of a cavity tray through the wall linked to a
membrane across the floor. This is then sealed to a 300 üm polyethene
membrane laid across the beam and block floor. To make it easier to seal the
two materials the cavity tray is laid so that it laps about 300mm over the
edge of the floor. The membrane over the floor can then be sealed to the
cavity tray using a double sided butyl jointing strip just prior to installing the
floor topping. Airbricks are installed where possible on all sides of the
building at intervals at least as frequent as would be normal for an ordinary
suspended timber floor.