Rates of reaction
GCSE COURSEWORK
CHEMISTRY
2004
AIM
In the experiment we use hydrochloric acid which reacts with the magnesium to form magnesium chloride. The hydrogen ions give hydrochloric acid its acidic properties, so that all solutions of hydrogen chloride and water have a sour taste; corrode active metals, forming metal chlorides and hydrogen; turn litmus red; neutralise alkalis; and react with salts of weak acids, forming chlorides and the weak acids.
Magnesium, symbol Mg, silvery white metallic element that is relatively unreactive. In group 2 of the periodic table, magnesium is one of the alkaline earth metals. The atomic number of magnesium is 12.
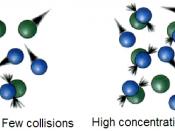
The rate of a chemical reaction is a measure of how fast the reaction takes place. It is important to remember that a rapid reaction is completed in a short period of time. An example of a fast reaction is an explosion, and an example of a slow reaction is rusting.
PLAN
*Equations:
Mg+2HCl MgCl +H
" "
Mg+2H Mg+H
"
The magnesium will react with hydrochloric acid, because it is higher in the reactivity series that hydrogen. When the two chemicals react, a displacement reaction will take place and the magnesium will displace the hydrogen in the hydrochloric acid forming magnesium chloride and hydrogen gas. Therefore, the products of the reaction are magnesium chloride and hydrogen gas.
For my experiment the results will be measured with a stop clock and the length of time the magnesium takes to dissolve will be measured. The results will be taken down in a table, and then a graph will be drawn from the information. The concentration of hydrochloric acid is calculated by moles and I will vary the concentration from 0.5M- 3.0M. Each time, a 1cm strip of standard width magnesium ribbon will be used. Each time...


Good exam essay
It's a goog essay.
3 out of 3 people found this comment useful.