Respiration is a process which releases energy from energy-rich molecules such as glucose. The glucose is converted into energy which is usable for life processes. Burning, or combustion, is a reaction between a substance and a gas to release energy. These two reactions have many similarities but are not the same. This essay will identify some of similarities and differences between the two processes.
The first similarity is that both respiration and burning use oxidation to release energy. In respiration, oxygen combines with glucose to release energy which can be used in the body. Also some heat is produced to keep the body warm. Burning normally occurs in oxygen (in form of O2) to form oxide. However, burning can take place in other gases such as chlorine. Also both of these reactions are exothermic - both reactions release heat after reacting with oxygen.
Another similarity is that both reactions release energy after oxidation.
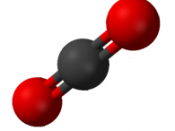
For respiration, the equation for the oxidation of glucose is:
Glucose + Oxygen â Carbon dioxide + Water + Energy
C6H12O6 + 6O2 â 6CO2 + 6H2O + energy
A substance called ADP (adenosine diphosphate) reacts with a phosphate group to form an ATP molecule (adenosine triphosphate).
ADP + phosphate + energy â ATP
In burning, the energy released is in form of heat and light.
The final similarity is that both reactions produce waste products. Respiration produces energy, carbon dioxide and water. Carbon dioxide and water are waste products as they are not needed in the body. In burning, the products include water as well as carbon monoxide (CO) or carbon dioxide (CO2), or both. Other by-products from reactions such as burning fuel and coal, may produce smoke and soot.
The first difference is that respiration needs sugar and oxygen (unless it's anaerobic respiration) to produce...