I liked "The Precious Envelope" because I learned about the importance of the atmosphere and ozone layer. It was interesting to listen to the different scientists and other important persons talk about the problems facing the depletion of the ozone layer and what must be done to save it. I want to write what the atmosphere and the ozone layers are and how we can help to save the ozone layer.
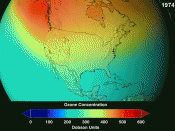
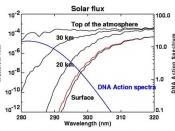
Atmosphere is a thin mass of gases over the Earth that controls the climate and weather conditions, in connection with the oceans. The atmosphere is made of these gases: nitrogen (78.1 %), oxygen (20%), argon (less that 1%), and other trace elements. The ozone layer is made of three oxygen atoms that block the sun's ultraviolet rays and is between 15 to 40 kilometers from the Earth. The ozone layer, or the "precious envelope", is under attack by its occupants and must be saved, or else life on Earth will die from overheating or freezing.
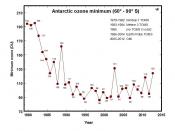
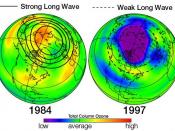
We are the cause for the depletion of the ozone layer with the many pollutants that are released into the air. According to Robert Watson, chief of atmospheric science at NASA, the major cause of ozone depletion is Chlorofluorocarbon (CFC), which is found in coolants for air conditioners, refrigerators, and cleaning solvents for computer components that rise into the atmosphere. When CFC's rise into the air, chlorine is broken off by UV rays and can breaks down thousands of ozone molecules. In the 1970's, scientists detected a hole in the ozone layer around the South Pole; and by 1985 the hole had doubled in size. In 1988, a study by NASA showed that the ozone had depleted significantly in the Northern Hemisphere. Now the question is what can we do about this growing problem that affects our future? Many companies that produce CFC's are working together to reduce the amount of these molecules by using alternative products. Robert Srubar, environmental coordinator in the Freon Division at Dupont Company, said that Dupont was developing alternative products, fluorocarbons, in which hydrogen and some chlorine give it stability and has a shorter time to live in the atmosphere. I feel that I can do my part by using other modes of transportation to go place to decrease the level of carbon monoxide that is taken into the air. I feel that the way scientists study ways in helping to save the ozone layer will give us clues into what we can do for our part.
Scientists use many different methods to figure how the ozone was first formed and how older atmospheric conditions affect us today. In the video, Cyril Ponnamperma, a research in the field of atmospheric sciences, used the "primordial soup" experiment to explain how the atmosphere started to help form life. He stated that 4.6 billion years ago, volcanoes released nitrogen gases in the air to produce the atmosphere, and water vapors condensed to evolve into the oceans. Lightening caused electrical discharges in the ocean to make organic compounds to start life. Other methods of scientists using to find what started the ozone include checking age rings of trees, radiocarbon dating of rocks in the ocean, and studies of the atmosphere today.
I learned a lot from this video concerning the ozone layer and the importance of it in our lives. I never realized just how important the ozone layer in blocking out the sun's UV rays and how much responsibility we really have in protecting it. As the video stated things like the greenhouse effect, carbon monoxide, and CFC's must be avoided if we want to live on this wonderful. I have a new sense of appreciation for this "precious envelope" and promise to protect it with my all power for the rest of my life.