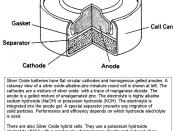
The silver button cell is commonly called a silver oxide battery. It consists of Zinc (Zn) the anode (-), Silver (Ag) the cathode (+) and a Potassium Hydroxide (KOH) paste is used as the electrolyte that contains free ions making the cell an electric conductor.
Cathode process:
Ag2O(s) + H2O(l) +2eï 2Ag(s) + 2OHï(aq) +0.80V
Eú = Eú Cathode - Eú Anode
0.80-(-0.76)= 1.56V
Silver is a rare element this means it is expensive, but when used in a button cell of a small size battery or an industrial size battery its high performances will easily make up for the cost. It can keep a wrist watch running for up 24/7 for 3-5 years
Advantages:
High capacity per unit weight
Constant voltage is produced
Low self discharge (long shelf life)
Heavy metal free (MG, Pb, Fe, Al, Be)
Help hearing impaired (keep there hearing aids lasting longer)
Disadvantages:
Poor low temperature performance
Expensive materials mostly for high performance applications
Limited cycle (not rechargeable)
Non- recyclable
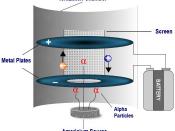
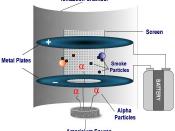
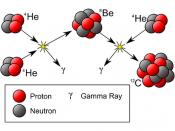
Americium 241-alpha particle is the radioisotope used in smoke detectors.
Americium-241 (Am) decays to produce Neptunium-237 (Np), Gamma rays and an alpha particle which has high ionization energy, causing the oxygen molecules in the air to ionize.
O2 2O+ + e-
Decomposition of Americium-241 241Am Np + ñ + ó
The positively charged particles, Alpha particles and positively charged Oxygen molecules, move to the negative electrode in the smoke detector. Negatively charged particles, electrons, move to the positive electrode.
The electrically charged ions carry a current from the negative to the positive. When smoke is present, Alpha particles attach themselves to the smoke which neutralizes them causing a drop in current, due to that the air molecules are no longer ionized. The smoke detector sensor sets of the alarm because of the drop in current.
When smoke detectors are thrown out they are transported to private landfills. Which then the Americium 241 decay chain ends with a stable non radioactive element bismuth 209
Formation of Americium
Americium 241 (Am) is an artificially made radioactive metallic element produced from Plutonium 241 (Pu). Plutonium 241 is formed in any nuclear reactor by neutron capture starting from uranium 238.
When Plutonium 239 absorbs two neutrons it produces plutonium 241, which by emitting Beta particles decays to become Americium 241.
Factories that use smoke detectors only have minimal amounts of Americium.
The alpha radiation is not strong enough to penetrate the plastics in the smoke detector but the gamma radiation will.
Exposure is unlikely unless you work with it in reactors
As americium decays, it releases alpha and gamma radiation and changes to neptunium 237 which is also radioactive
Americium 241 is an unstable radioisotope with a half life of 433 years.
Decay - ò
Standard Cell Potential