Definitions
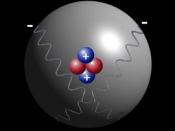
Atom- An atom is a very small piece of matter. These tiny particles are the entire matter of the universe. An atom consists mainly of space and is composed of tiny particles of matter. An atom is composed of three types of matter Protons, Neutrons and electrons.
Nucleus- The dense positively charged center of an atom.
Proton (P+)- Located in the nucleus is a positively charged particle called a proton.
An atom has an overall positive charge when it loses electrons from its valence shell in chemical reactions.
Neutron (N)- Neutrons reside in the nucleus with protons and have a neutral charge.
The number of Neutrons determines the isotope of the atom.
An isotope is an atom with the same amount of protons in its nucleus but a different number of neutrons in its nucleus than it would normally have.
Electron (e-)- Orbiting the nucleus at high speeds are the electrons.
Electrons are electrically negative and about 1/2000 the mass of Protons or Neutrons.
An atom has a overall negative charge when it gains electrons to fill its valence shell during a chemical reaction.
Name Location Charge Mass Symbol
Proton Nucleus Positive 1 P+
Neutron Nucleus Neutral 1 N
Electron Electron Cloud/Shell Negative 1/2000 e-
Atomic Number- The atomic number is the number of protons in an individual atom. It is also the number of electron in a neutral atom.
Atomic Mass- The atomic mass is the sum of protons and neutrons.
You can figure out the number of electrons by deducting the atomic number from the atomic mass.
Atomic Symbol- The atomic symbol is like a notation of an atom. It is used for convenience. It is usually the first letter of an atom (It can also include the second or the third letter). It enables quick notation...


Good notes
Good notes, very useful.
2 out of 2 people found this comment useful.