Ionization energy: decreases when going down the group because when going down the group the number of shells around the atomic radius increases and the outer electrons become weakly attracted by the nucleus.
As you go to the rite the no. Of neutrons in the nucleus (+) charged particles increases. This pulls the electrons towards the nucleus. Therefore making it more difficult to remove the electrons.
Electro-positively: increases down the group e.g. Potassium is more electropositive that sodium and decreases along the period from left to the right.
Electro-negatively: decreases down the group and increases along the periods from left to right.
Metallic character: going down the group the metallic character decreases (non-metallic) character decreases down the group.
Atomic radius: going down the group the atomic radius increases because the atomic radius decreases along the period from left to rite.
Reactivity: going down the group 1 the reactivity of the elements increases while going down the group 7.
The reactivity of the elements decreases.
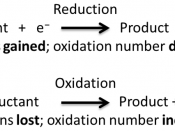
Redox reaction: reduction and oxidation reactions are known as redox reactions because they take place simultaneously (at the same time).
Reduction (redactant): is the removal of oxygen from a substance, the addition of hydrogen to a substance, the addition of electrons to a substance.
Oxidizing agent: is a substance which adds oxygen from another substance, removes hydrogen to another substance, removes electrons to another substance.
Oxidation: (oxidant) is the addition of oxygen to a substance, the removal of hydrogen from a substance, the removal of oxygen from a substance.
Bonding: the process whereby two atoms are joint together to form one atom.
Bond: the process whereby a strong force of attraction holding the atoms together.
Ionic bonding: is the type of bonding formed between 2 atoms or more after one of them loses electrons and...