Just twelve miles from Harrisburg, Pennsylvania, a nuclear plant by the name of Three Mile Island became unstable. It was March 28, 1979. It was only 4 o'clock in the morning when the small malfunction occurred. What only started as a small error soon developed into problem of much publicity. There were many details that went into the accident, including nuclear chemistry. Many things were involved in the nuclear accident at Three Mile Island, but in the end catastrophe was prevented.
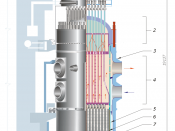
Inside Three Mile Island, a process called nuclear fission is a work. This is but one of two nuclear reactions that are made. The other reaction is called fusion, or the combining of nuclei. In fission, atoms are split apart to make energy. This fission produces power, heat, and electricity. In a nuclear plant, the use of fission is very similar to a coal or oil-powered plant by the fact that they all use heat as power.
The only difference lies in how the heat is generated. Nuclear chain reactions produce heat by using isotope, or uranium element. Uranium pellets, or U-235, are inserted into the core of a reactor to create the energy. Why use uranium? The uranium is constantly decaying into loose neutrons, which move very quickly. These neutrons collide with other uranium pellets, causing heat and kinetic energy. This is the process of nuclear fission.
Control over all this energy is a major key to managing such power. Control rods are used just for this purpose. Also known as shims, these control rods are made of cadmium and boron elements. The elements in the rods are used to slow down the movements and fast pace of speeding neutrons. If the heat of neutron movement gets too high, the neutron-absorbing rods are thrust into the reactor,
![Singer. Anti-nuke rally in Harrisburg, [Pennsylvania] at the Capitol., 04/09/1979](https://s.writework.com/uploads/10/104544/singer-anti-nuke-rally-harrisburg-pennsylvania-capitol-04-09-thumb.jpg)