Abstract
Water is essential for all living organisms. The physical and chemical properties of water are central to all biological structure and function. The ionization ability of water to form H+ and OH- ions make it very unique. The hydrogen-ion concentration of biological system is usually preferred as the pH system, which determines the pH level of dilute aqueous solutions. In this laboratory exercise, the data collected from the titration of weak acids with a strong base will be used to graph the titration curves and these titration curves will be used to identify an unknown acid by comparing its dissociation constant with dissociation constant of other known acids.
Introduction
The effects of pH and buffers play an important role in the protonation and deprotonation of water. Many organisms survival depend on the pH levels; therefore, the important of understanding of the response of water to other aqueous solutions is critical.
In this experiment, we titrated Acetic, Glycin, Histidine, Glutamic acids and an unknown acid with potassium hydroxide (KOH).
Materials and Methods
The pH meter was standardized with the pH 4 for all five titrations. Five 100 ml beakers were prepared and the amount of 40 ml of 0.05M Acetic acid, 0.05M Glycin, 0.05M Histidine, 0.05M Glutamic acid, and an unknown acid were added to each beaker. The initial pH reading was recorded for each titration and the KOH solution was added by increment of 1 ml and during the titration, the pH was recorded when the meter indicated that the electrode had reached a steady value. Each of the titration stopped after a total of 19 ml of KOH have been added to the beaker.
Results
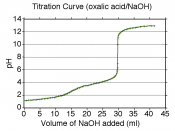
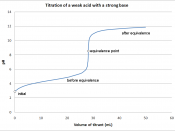
We titrated Acetic acid and recorded all the data. The total solution of KOH added was 19 ml. The titration curve of...