There's a place called Uranium That just can't be beat From preserving our food To giving us heat This place called Uranium Helps us people a lot Even though it is tiny, Much smaller than a dot If you've never heard of Uranium Well, you're in for a treat So just sit back, relax And I'll tell you something neat About this place called Uranium That's really pretty cool In fact it's much like your Town, church, or school There are a couple things though That you'll have to get used to Before you understand Uranium And be part of the crew The words in Uranium May seem different and scary The boys are called electrons Unlike you, me, and Larry The girls in Uranium Have a different name as well For they are called protons, And it suits them quite swell.
After the protons and electrons Grow up and go steady They can go off and get married, If they think they are ready After this happens They are called something new For they are now neutrons, A combination of the two They then have to move Into downtown Uranium, Nucleus is what it's called, Not to be confused with titanium.
Within the town of Nucleus The neutrons are not alone For there are many neutrons there With whom to shop and talk on the phone.
146 neutrons Are usually here Although the number can change It's usually near All of these neutrons Are raising their babies, Trying to teach them And protect them from rabies The 92 protons stay near their neutron And inside of their bubble, While the electrons run wild and cause all sorts of trouble.
The 92 electrons run around And circle Nucleus all day, Much to their Neutrons agony and dismay.
It's hard to blame them Because they are so young It's hard to stay in And not have any fun But sometimes their neutrons Cannot find where they are Due to the empty land Around Nucleus that is very far.
Uranium is an element, And in case you wern't aware Elements are the building blocks Of the sea, land, and air.
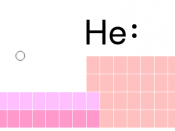
On the periodic table Located to the right It's the element U, To it's great delight.
Shown also on the table Are it's atomic number and mass Notice that U is very heavy, All natural metals does it pass Uranium is not perfect though, And it has many flaws, As I will tell you about, In my next little clause.
Sometimes an unwanted neutron That likes to yell and shout Causes the city of nucleus To divide and explode out This starts a chain recation That we like to call fission And it creates tons of energy, Just like a magician.
This process is quick; Done with simplicity And it is what we use To make electricity.
Called nuclear energy, It is something quite new And is a topic of debate For more then a few When Uranium was discovered Over two hundred years ago The process of nuclear fission We did not yet know Martin Klaproth named it after the planet Discovered around then Never knowing what good It could bring to men But now we know Uranium Inside and out And so now do you, Without a doubt.
Glossary 1)Element - Elements are found on the periodic table and are atoms with a certain amount of protons.
Uranium is an element with 92 protons.
2)Atomic Mass - The atomic mass of an element is the average weight of all the types of an element.
Since there are different types (isotopes) of the same element, they add all of these together to find the average.
3)Atomic Number - The atomic number of an element is the number of protons that are in the element.
4)Isotope - Isotopes are variations of elements. They have more or less neutrons then the normal atom does.
5)Compound - A compound is a combination of two or more elements together.
6)Electron Cloud - The electron cloud is the vast area that surrounds the nucleus of an atom. This area is where the electrons dart all around.
7)Atom - Atoms are the tinybuilding blocks of everything in the whole world.
8)Protons - Not actually girls, they are tiny positively charged particles within an atom.
9)Electrons - Not actually boys, electrons are almost weightless tiny particles with a negative charge.
10)Neutrons - Not actually parents, Neutrons are what happens when protons and electrons join together.
11) Fission - Not saved.