Steven Tran CHM111B Mrs Lewis
Water EEI
Abstract
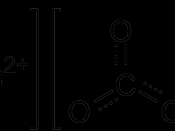
This experiment involves the determination of the concentrations of the calcium ion and magnesium ion from 10 sources of water, 5 bottled and 5 tap. This is accomplished by a titration method using a chelating agent called EDTA, which is short for, ethelynediamine tetraacetic acid. The first method is a titration using Eriochrome Black T as indicator determines total hardness due to the calcium and magnesium ions. The hardness due to the calcium ion is determined by a separate titration at a higher pH, which is done by adding Sodium Hydroxide solution to precipitate magnesium hydroxide, using hydroxynaphthol blue as indicator. Many variables will have to be taken into account when considering the results of this experiment. Such variables can include inconsistencies with equipment or the temperature of the lab. The data found from this experiment shows that the hardness of tap water in Brisbane is consistent, with minor issues and bottled water hardness varies accordingly to brand.
Introduction
Ever wondered what that strange coloured solid on your tap is, and why it is caused? Also found on kettles, it is called limescale, and is the result of what is called 'hard' water evaporating on the surface of the object. Hard water results in many other solids similar to this being formed, such as a very common one, which is informally called 'soap scum'. Although one may think that such a minor thing would be harmless, in fact, there have been many reports where soap scum found on vinyl shower curtains constitute a rich microbial biofilm, containing potentially pathogenic bacteria.
Originally the hardness of water was thought to be a measure of the capacity of water for precipitating soap. The term hard water comes from the fact that these metal ions precipitate...