Werner syndrome (WS) is a rare autosomal recessive disorder characterized by premature aging (von Kobe, et al., 2003). It is named after the German physician Carl W. Otto Werner (1879-1936), who first described the syndrome as part of his doctoral thesis in 1904. WS is caused by mutations in the RecQ family of helicase which are encoded by chromosome 8p by the WRN gene (Moser, et al., 1999). The mutations truncate the WRN protein with a loss of up to 1256 amino acids. In other words, WS is caused by a helicase defect, and as a result, DNA replication is impaired. WS syndrome is mainly characterized by rapid aging beginning at adolescence and resulting in old age by the age of 30 or 40. The physical characteristics of WS are short stature, hoarse high-pitched voice, juvenile bilateral cataracts, premature graying of hair, skin changes, diabetes, cancer and other diseases found in the elderly (Faragher, et al.,
1993).
Werner syndrome greatly decreases the replicative life-spans of fibroblasts (cells that give rise to connective tissue). Normal fibroblast cells double about 60 times in vitro (an artificial environment, i.e. culture) while WS cells only double about 20 times in vitro (Faragher, et al., 1993). From this evidence, Faragher hypothesized that the WS gene is a "counting gene," meaning that it controls the number of times the cells are able to divide; cells affected by WS start of like normal cells but are eventually terminated by the counting gene after a number of replications.
The two possible reasons for the decreased life span of WS cells are that, the cells start out normal and eventually decline in reproductivity due to WS, or that there are a few number of cells to begin with, and they lose their reproductivity at a normal rate (Faragher, et al., 1993). To test these two hypotheses Faragher examined the behavior of fibroblast cultures from three WS patients and three normal control strains. The results show that WS cells and normal cells began with the same reproductive rate, but the reproductivity of WS cells dramatically decreased. Faragher was able to determine this by measuring the number of cells which where in the S phase. Faragher also concluded that WS gene was a "counter" which controlled the frequency at which cells could leave the cell cycle (Faragher et al., 1993). He concluded this because in the absence of WS gene function, the cell cultures still exited the cell cycle as they normally would do. This could only mean that the WS gene controlled the senescence of the genes, and in turn acts as a counter to determine which cells retire from the cell cycle
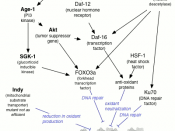
It is not yet determined whether the WS transcription defect is global or localized to certain genes and the role of WRN in transcription remains elusive (Kyng, et al., 2003). With this Kyng set up trials to study 6,912 RNA pol II transcribed genes in a panel of 15 different human fibroblast cells derived from both normal and WS patients, to determine if WS is specific to certain genes. Of the 6,912 genes tested, only 6.3% of them showed significant differences in their expressions, when cells from either WS or old donors were compared with young normal donors. The results show that the pathways involved in generating WS and aging are very similar.
In another experiment, von Kobbe determined that poly(ADP-ribose) polymerase 1 (a nuclear enzyme which protects the genome by facilitating DNA repair) was absent in WS cells (von Kobbe, et al., 2003). This enzyme responds to DNA damage by transferring 50 to 200 molecules of ADP-ribose to various nuclear points (von Kobbe et al., 2003). Poly(ADP-ribosyl)ation activity is important in maintaining the genome and is also associated with longevity. The results of the experiment concluded that poly(ADP-ribose) polymerase 1 is active in WS cells but its ability to ribosylate proteins after DNA damage is severely hampered (von Kobbe, et al., 2003).
The conclusions of all three experiments are not concrete. More studies and experiments need to be done to fully understand Werner syndrome. Faragher's experiment sought to prove that the gene responsible for WS was actually a gene which controlled senescence and he was right. He concluded that WS gene is a counter that modulates the frequency at which cells in culture leave the cell cycle (Faragher, et al., 1993).Still, there is much more research to be done to understand all aspects of the gene. Kyng's experiment focused mainly on the types of genes which were affected, but more research is needed before his findings could be considered definite.