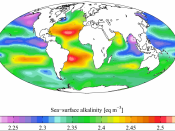
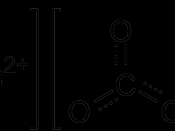
Alkalinity is "the water's capacity to resist changes in pH that would make the water more acidic." (Wilkes). The overall purpose of alkalinity is to protect the body of water from quick changes in pH levels. However, alkalinity is not to be confused with pH. pH is how intense the basic or acid state of a liquid is. pH is determined by the alkalinity of the water. Alkalinity is a buffer, which means it can neutralize ions. Alkalinity neutralizes hydrogen and hydroxide ions specifically. The majority of alkalinity comes from calcium carbonate, which dissolves when water moves through soil or over rock. It also comes from bicarbonate and hydroxide ions. Hardness is also highly associated with alkalinity. This is because the bulk of alkalinity comes from limestone which contains a great deal of calcium carbonate. Hard water includes mostly calcium carbonate, similar to alkalinity, making the two essentially equal.
Alkalinity is essential for fish and other marine life because it shields them from quick pH differences. The best pH level for marine life is between six and nine. The higher the alkalinity, the more effectively it will buffer acid rain and shield against pH variations. When the alkalinity buffers the acid, it can leave the water with low alkalinity levels. This can temporarily lower the pH levels which can be harmful to the marine life. On the other hand, high alkalinity levels can also be harmful. In a watershed, if the water has too much alkalinity calcium and magnesium levels can build up in the soil and block the plants from soaking up the water. High alkalinity is not usually harmful to marine life.
An example of high alkalinity in the world today is in Panaji, India. The problem started out as the water producing an unpleasant smell and has now resulted in high pH. After multiple tests, the water has been confirmed to have a pH of over seven. This could "lead to dysentery, vomiting, diarrhea." (Pereira). This high alkalinity has cause many establishments to close and has greatly effected the economy in Panaji. This is one of many examples of the negative effects of alkalinity on our watersheds.
Works CitedPereira, Andrew. "Alkaline water baffles PWD, Panaji residents suffer." The Times of India. 19 Sep 2008. India Times. 28 Sep 2008 .
Cox, Douglas. "Greenhouse Management." Floriculture. Aug 1995. University of Massachusetts. 28 Sep 2008 .
Wilkes University, "Alkalinity- The Protector of the Stream." Wilkes University Center for Environmental Quality Environmentmental Engineering and Earth Sciences. N.A.. Wilkes University. 28 Sep 2008 .
![[Map Showing Chlorine, Hardness and Alkalinity of Ground Water North of Winnipeg] (1907)](https://s.writework.com/uploads/13/135009/map-showing-chlorine-hardness-and-alkalinity-ground-water-n-thumb.jpg)