For the last hundred years, gasoline powered vehicles have ruled the streets. The oil industry is a major player in the world marketplace and the health of the environment as a result of car pollution and the United States' dependence on foreign oil remain hot political issues. As of yet no viable alternative has entered the marketplace to attempt and unseat oil's hundred-year monopoly. Fuel cells promise to be the next generation in fuel technology, offering better and more environmentally friendly designs.
The best way to describe a fuel cell is as a battery; both create energy via silent electrochemical reactions. A fuel cell doesn't need recharging and can run indefinitely as long as it gets more fuel. Fuel cell technology has been around since the 1960's but due to its cost and size has only been used on spaceships and more recently as backup generators in places such as hospitals and office buildings.
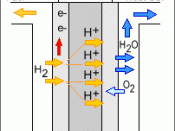
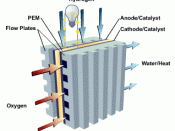
There are four main components of a fuel cell the cathode, anode, electrolyte, and catalyst. The cathode is the positively charged section of the fuel cell. The cathode has channels that run through it that distribute the oxygen to the surface of the catalyst. The cathode also conducts the electrons back from the external circuit to the catalyst, where they can recombine with the hydrogen ions and oxygen to form water. The anode is the negatively charged section of the fuel cell. It conducts the electrons that are freed from the hydrogen molecules so that they can be used in an external circuit. The anode also had channels running throough it that disperse the hydrogen gas equally over the surface of the catalyst. The electrolyte is the proton exchange membrane. This is a specially treated material which usually looks something like ordinary saran plastic wrap. The...

Alternative fuels, fuel cells
Good report. Very interesting with alot of information.
0 out of 0 people found this comment useful.