Pure aluminium is a relatively soft, silvery white metal. When exposed to air, a thin coating of Aluminium oxide gives it a dull lustre. It is three times less dense than water, and has great strength when alloyed, it doesnÃÂt rust and has high electrical conductivity, and Aluminium is also ductile, making it a very useful metal. Aluminium readily makes alloys with copper, zinc, magnesium, manganese and silicon. Aluminium foil is 92-99% pure aluminium. Other uses of aluminium alloys include computers (all Apple MacBooks are made from aluminium), cooking utensils, transportation (aircraft, rockets and cars mainly because of their high strength-to-weight ratio), packaging, water treatment, street lighting etc.
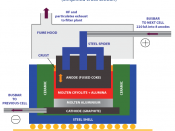
Aluminium is the most abundant metallic element in the lithosphere, thought to be 7.5 to 8.1%, it is rare to find it isolated. Due to its high reactivity it forms a high-energy bond with oxygen, thus making it difficult to extract. Therefore it has to be refined form alumina, using the Hall-Heroult process (below).
Alumina is produced by the Bayer Process from bauxite and used in the production of aluminium metal. It is also used as a refractory material, which is a material which keeps its strength even at high temperatures. These materials are usually used in linings of furnaces and kilns, incinerators and reactors.
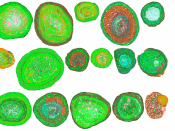
Bauxite is an orange-red igneous rock, which occurs naturally in the lithosphere. It contains 30-54% alumina, Al2O3 and other impurities such as clay, Iron (III) Oxide (Fe2O3), Silica (SiO2), and Titania (TiO2). Australia was the top producer of bauxite in 2007, with almost one-third world share.
The Bauxite has to be purified by a process known as The Bayer Process. The Bayer process is the main industrial method of refining bauxite to produce alumina. In the process the bauxite is digested by mixing it with a hot...