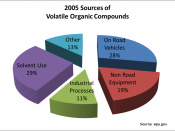
Paint the objectPaints have a particularly distinct effect on environment and health. Paints are a major source of indoor air pollution. The US Environmental Protection Agency puts paint on its top-five list of environmental hazards. Conventional paints can make indoor air a chemical cocktail, even long after they have dried, as they continue to release petroleum based solvents, called Volatile Organic Compounds (VOCs) as they cure.
Studies have found that the cumulative VOC emissions from architectural painting operations exceed the combined emissions from a variety of industrial operations. VOCs from solvent and paint emissions contribute to harmful ozone formation and peroxyacetyl nitrate.
According to the Masters Painters Association, ozone from paint emissions irritates eyes, nose, throat and lungs; reduces breathing capacity even in healthy adults and children; increases susceptibility to infection, hospital visits and admissions; and causes damage estimated to cost over millions of dollars per year to crops and buildings.
Another problem with synthetic paints is post-application wastage and disposal. The petrochemical paints that currently dominate the market are predominately derived from oil, a non-renewable resource.
Waste needs to be specially treated to avoid adverse environmental impacts. It has been estimated that water-soluble gloss paints require dilution of 40m to 1 to render their entry to the sewerage system harmless.
ÃÂMany of the larger paint companies have produced products which have been certified to be 'environmentally friendly', but are still synthetic paints made from petrochemicals, with lower VOC concentrations. However, these products are still a step in the right direction, and should be considered by specifiers and consumers who wish to use acrylic paintsÃÂ, Wurm said.
Cover the iron with a thin coating of tinThe influence of inorganic tin compounds on the unicellular cyanobacterium Synechocystis aquatilis was studied, and its dependence on changing pH of the surrounding medium and the presence of humic acid. Both Sn(II) and Sn(IV), used as chlorides (at the concentrations 1ÃÂ10 mg litreâÂÂ1), inhibit the growth and chlorophyll a content of the cyanobacterium cultures, but only under alkaline conditions. Generally, the observed tin toxicity increased with increase of metal concentration, time of exposure and pH value of the medium (in the range 7ÃÂ9.8). Sn(II) seems to be more toxic than Sn(IV). At the lowest studied metal concentration (1 mg litreâÂÂ1), Sn(II) caused a 36 and 40% decrease in growth and chl a content, respectively, after 96 h exposure at pH 9.8, while Sn(IV) caused even a slight increase of both physiological parameters (hormetic effect). Similar increases in growth and chl a content were also observed at a high Sn (II) and Sn(IV) concentration (10 mg litreâÂÂ1), but only in cultures exposed to metal at pH 7. At high pH (9.8), 10 mg litreâÂÂ1 of Sn(II) and Sn(IV) significantly suppressed both the growth of the cyanobacterium (by 54.2 and 26.1%, respectively) and the chl a content in cultures (by 58.2 and 24%, respectively). Humic acid reduced the toxicity of tin towards the cyanobacterium. The observed effects of pH and complexing ligand on the inorganic tin toxicity are discussed in the context of changing, chemical metal speciation and bioavailability. (http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6VB5-3T7CHRM-8&_user=10&_rdoc=1&_fmt=&_orig=search&_sort=d&view=c&_acct=C000050221&_version=1&_urlVersion=0&_userid=10&md5=ae254bdd7238ca45a2d8d2ec16455091)