Asymmetric Epoxidation of Dihydronaphthalene with a Synthesized Jacobsen's Catalyst
Justin Lindsey
12/08/96
Chem 250 GG
Professor Tim Hoyt
TA: Andrea Egans
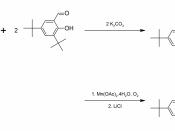
Abstract. 1,2 diaminocyclohexane was reacted with L-(+)-tartaric acid to yield (R,R)-1,2-diaminocyclohexane mono-(+)-tartrate salt. The tartrate salt was then reacted with potassium carbonate and 3,5-di-tert-butylsalicylaldehyde to yield (R,R)-N,N'-Bis(3,5-di-tert-butylsalicylidene)-1,2-cyclohexanediamine, which was then reacted with Mn(OAc)2*4H2O and LiCl to form Jacobsen's catalyst. The synthesized Jacobsen's catalyst was used to catalyze the epoxidation of dihydronaphthalene. The products of this reaction were isolated, and it was found that the product yielded 1,2-epoxydihydronaphthalene as well as naphthalene.
Introduction
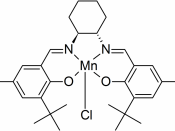
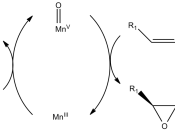
In 1990, professor E.N. Jacobsen reported that chiral manganese complexes had the ability to catalyze the asymmetric epoxidation of unfunctionalized alkenes, providing enantiomeric excesses that regularly reaching 90% and sometimes exceeding 98% . The chiral manganese complex Jacobsen utilized was [(R,R)-N,N'-Bis(3,5-di-tert-butylsalicylidene)-1,2-cyclohexanediaminato-(2-)]-manganese (III) chloride (Jacobsen's Catalyst).
(R,R) Jacobsen's Catalyst
Jacobsen's catalyst opens up short pathways to enantiomerically pure pharmacological and industrial products via the synthetically versatile epoxy function .
In this paper, a synthesis of Jacobsen's catalyst is performed (Scheme 1). The synthesized catalyst is then reacted with an unfunctional alkene (dihydronaphthalene) to form an epoxide that is highly enantiomerically enriched, as well as an oxidized byproduct.
Jacobsen's work is important because it presents both a reagent and a method to selectively guide an enantiomeric catalytic reaction of industrial and pharmacological importance. Very few reagents, let alone methods, are known to be able to perform such a function, which indicates the truly groundbreaking importance of Jacobsen's work.
Experimental Section
General Protocol. 99% L-(+)- Tartaric Acid, ethanol, dihydronaphthalene and glacial acetic acid were obtained from the Aldrich Chemical Company. 1,2 diaminocyclohexane (98% mix of cis/trans isomers) and heptane were obtained from the Acros Chemical Company. Dichloromethane and potassium carbonate were obtained from the EM Science...