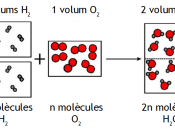
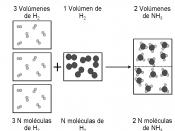
Today, we describe Avogadro's number as 6.02 x 10^23. It was not always taken for granted that this number was valid. In 1811, Amedeo Avogadro proposed his hypothesis at a time when there was no data on the amount of particles in a mole.
Avogadro's constant (Na) was estimated early by the number of molecules in a given volume as well on molecular diameter by Loschmidt. The first experiment designed to test Avogadro's hypothesis was conducted by Robert Brown in 1827. This experiment generated an approximate value of Avogadro's number. Shortly thereafter, in 1860 Cannizarro used Avogadro's hypothesis to develop a set of atomic weight, weighing 1/16 that of an oxygen atom. Over the next one hundred years, more accurate estimates of Avogadro's number were made.
By 1933, there was no consensus on what the number should be called. After experiments and analytical research, it was agreed upon that Avogadro's Constant would be an appropriate name, since it was in fact Amedeo Avogadro who came up with the initial hypothesis.
The best modern values for what we today call Avogadro's constant is a result of x-ray diffraction measurement of lattice distances in metals and salts. It is also called x-ray crystallography, and this procedure arrives at precise dimensions in crystals with an error of less than .000 000 01. By 1958 Avogadro's constant was being used in school textbooks, a testament to the finally accepted number that has taken over 140 years to be recognized internationally.