The Biological Importance of Water Water is a liquid that is colorless, transparent and odorless. It is one of the most important biological systems and it has many unique properties that make it essential to all life. Water exists in nature in all three states of matter (solid, liquid, gas), and it also covers 75% of the earth and composes roughly 78% of our body. Most of water's unique properties are a result of the hydrogen bonds between water molecules.
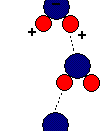
Structure of Water1. Hydrogen bonds because water molecules are polar covalent molecules, it has a slight positive and slight negative charge on opposite ends. Like the positive and negative ends of a magnet, the water molecules are attracted to each other. Because of these attractive forces, water molecules are in close proximity to one another, making water very dense. In addition, as a result of the attractive forces, the oxygen and hydrogen atoms form hydrogen bonds.
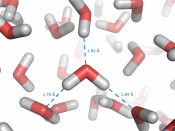
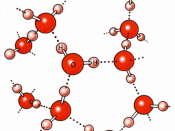
Since the oxygen atom has two pairs of nonbonding electrons, each oxygen in a single molecule can form H-bonds with two hydrogens. Figures on the next page show such a hydrogen bond. The resulting clusters of molecules make water cohesive. In its liquid phase, H-bonds are distorted and the network of molecules is irregular. When water freezes, the H-bonds form the water molecules into a regular lattice with more room between the molecules than in liquid water; therefore ice is less dense than liquid water.
2. Water as a solventWater is a good solvent. As a polar covalent compound, water can dissolve many substances. It is in fact called ÃÂthe universal solventÃÂ. Because polar covalent compounds have charged poles, they dissolve in water and so they are said to be hydrophilic, which means ÃÂwater lovingÃÂ. On the other hand, nonpolar covalent compound...