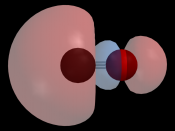
Carbon monoxide, written with the chemical formula CO, occurs as a gas found naturally in the atmosphere as well as created by man. It has a density of 1.161 kg/m3 in gas form at 70úF, barely less dense than that of air (approximately 1.202 kg/m3 at 70úF). CO is colorless and odorless, but is flammable and burns in air with a characteristic blue flame, producing carbon dioxide. Carbon monoxide has a boiling point of -192 úC (81 K) and a melting point of -205 úC (68 K). CO has a linear geometry and three resonance structures, two of which violate the octet rule. (Penny) Carbon monoxide is also extremely poisonous to all warm-blooded animals and to many other forms of life. Even .1% in the air is enough to kill a person within 2-4 hours, while 1% in the air can kill a person in under 5 minutes. When inhaled it combines with hemoglobin in the blood, preventing absorption of oxygen and resulting in asphyxiation.
It is a reducing agent, removing oxygen from many compounds and is used in the reduction of metals, such as iron, from their ores. (Penny)
Several major cycles and components in the atmosphere are affected by the release of carbon monoxide. One of the major issues with carbon monoxide in the atmosphere has to do with its relation to greenhouse gasses; components of the atmosphere that contribute to the greenhouse effect which warms the planet. (Reay) One such issue is with hydroxyl (OH) radicals. The hydroxyl radical is the neutral form of the hydroxide ion. Hydroxyl radicals are highly reactive and consequently short lived, and are often used as a "detergent of the troposphere" (Uherek) due to it's oxidation of several pollutants, including CO. Carbon monoxide in the atmosphere can also lead to the formation...