Alzheimer's disease
1) Primary Source - "FAD Mutations in Presenilin-1 or Amyloid Precursor Protein Decrease the Efficacy of a y-Secretase Inhibitor: Evidence for Direct Involvement of PS1 in the y-Secretase Cleavage Complex"
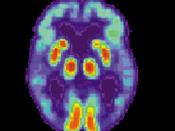
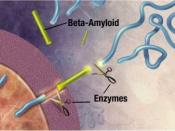
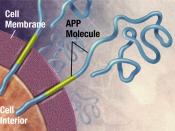
Alzheimer's disease (AD) causes mutations in presenilin-1 (PS1) and presenilin -2 (PS2) that increase the formation of amyloid B-peptides (AB). AB is formed by increasing the cleavage of B-amyloid precursor protein (APP), and the cleavage of APP is performed by y-secretase. So in simpler terms, AB peptides are formed by the cleavage of APP by B- and y- secretase. In order to examine whether PS1 is directly involved in the y-secretase cleavage complex, compound 1 was used to imitate the y-secretase cleavage region of APP, which would inhibit the AB generating system.
This experiment proved that peptidomimetic inhibitors such as Compound 1 as well as aspartyl protease inhibitor pepstatin A could inhibit the activity of y-secretase, which would decrease AB production, and as a result there is a surplus of y-secretase substrates, and this suggests that there is direct contact between the inhibitors and the active site of y-secretase.
It was found in this experiment that FAD (familial Alzheimer's disease) causing mutations in APP or PS1 decreased the capacity of compound 1 to inhibit the cleavage of y-secretase. The results of the experiment conclude that PS1 is directly involved in the cleavage of APP by y-secretase that results in the production of AB peptides. This study showed that despite previous studies that claim that the increase of AB production is due to FAD that causes mutations in PS1 and PS2, the mechanism that causes this increased cleavage by y-secretase couldn't be determined. This study also confirmed that: 1) that their finding an optimal AB synthesis at a slightly acidic pH and 2) the inhibition of AB generation...