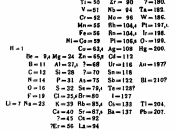1.1 The development of the periodic table
Atomic number: the number of positive charges or protons in the nucleus of an atom of a given element, and therefore also the number of electrons normally surrounding the nucleus.
Atomic Mass: the mass of an isotope of an element measured in units formerly based on the mass of one hydrogen atom taken as a unit or on 1/16 the mass of one oxygen atom, but after 1961 based on 1/12 the mass of the carbon-12 atom.
Isotope: either of two or more forms of a chemical element with the same atomic number but different numbers of neutrons.
Mass number: the number of protons and neutrons in the nucleus of an atom of a particular substance.
Radioisotope: a particular form of a chemical element (isotope) that is radioactive
Specific compounds we make are useful not because of what they produce, but because of the properties they have.
Thus the reason we use iron oxide (fe2o3) in videotape, floppy disks, and hard drives. The properties such compounds as this form depend on their location in the periodic table. This is not what the best part of chemistry is, as we already know these things, but most exciting is what we have yet to discover, such as a compound that will conduct electricity without resistance at room temperature. This would enable faster computers, better MRIS and levitating mass transit vehicles, all would also have a substantially lower energy use.
The organization of elements
It was realized that each element had a characteristic mass in the 18th century. Hydrogen was given the atomic mass of 1 because it was the element with the lowest mass, all other elements thereafter were compared to that of hydrogen, this gave a relative atomic mass for each element. As each...