Formation of Compounds: The Existence of Minerals1.Almost all the Elements in the EarthÃÂs Crust are found in the form of Compounds.
2.Only a few Metals such as Gold, Silver and sometimes Copper are found as free Elements.
3.Minerals are natural Elements or Compounds which have exact/precise/accurate and fixed/constant chemical composition/content.
4.The above definition of Minerals can differentiate Minerals from Rocks which contain various types of Minerals.
5.Examples of Minerals which exist in the form of free Elements include Diamond(Carbon), Gold, Platinum, Silver.
6.Most Minerals which exist in the form of Compounds include the Oxides, Sulphides and Carbonates of Metals.
e.g.
MineralChemical Name/CompositionChemical Formula1.HematiteIron (III)OxideFe2O32.CassiteriteTin (IV) OxideSnO23.BauxiteAluminum OxideAl2O34.PyriteIron (II) SulphideFeS5.GalenaLead (II) SulphidePbS6.LimestoneCalcium CarbonateCaCO37.MalachiteCopper (II) CarbonateCuCO38.MagnesiteMagnesium CarbonateMgCO39.EpsomMagnesium SulphateMgSO410.FluoriteCalcium FluorideCaF27.The formation of Compounds in their Natural state is based on the stability of Chemical Bonds.
8.Chemical Bonds are strong Electrostatic Attractive Forces which exist between Atoms which will enable the Atoms to exist in the form of Clusters, consequently forming Compounds.
9.The type of Bond which is formed depends of the type of Element or the type of Particles which are involved in the formation of the Compound.
Chemical Bonds*Electrostatic Attractive Force ÃÂ attractive force which exists between particles with opposite charges.
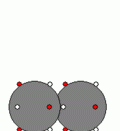
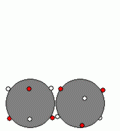
Examples of Electrostatic Attractive Forces:1. Metallic Bond2. Ionic Bond3. Covalent Bond1. Metallic BondStructure of Metal:-Many electrons are attached to each Cation by Metallic Bonds.
-Metallic Bonds are also between electrons and Cations in different layers of Metal.
-When a force is applied to the top layer of metal atoms, it will only ÃÂslideÃÂ a short distance but not all the Metallic Bonds are broken; some are retained to hold the two layers of metal together, allowing the metal to change its shape.