Abstract
Iron and steel has helped human beings build stable structures and craft solid tools.
But how is steel and iron made?
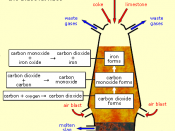
This report will examine the processes involved in producing steel and wrought iron starting with the extraction of iron ores from the Earth. This report will show how iron ore is refined into steel through oxidation occurring in several types of furnaces.
Introduction
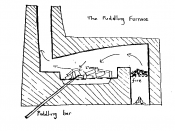
Steel and iron are well known metals that play a vital role in today's world. Wrought iron was originally used as the world's main building block but is now replaced by steels. Wrought iron is the commercial term used to describe pure or virtually pure iron. This report will cover the refining processes of wrought iron as well as carbon based steels.
Steel has come to replace wrought iron in many small scale and large scale situations. This is due to the effectiveness of modern day furnaces.
Bridges, nuts and bolts, girders and tools are all examples of items that were previously made of iron that are now made of steel. Steel itself is not an element but rather the resulting alloy of iron and carbon.
An alloy is a combination of two or more elements where at least one is a metal. The resulting compound is usually a stronger or more useful metal. There are various alloys of iron and carbon. They vary from having almost no carbon to having up to 5% carbon in it. The carbon effectively acts as a 'glue', making the iron hold together better. Unfortunately it makes it brittle and harder to work with.
Several different variations of iron and carbon alloys are listed below.
Carbon content | Alloy |
Less than 0.15% carbon | Wrought iron (pretty much PURE iron) |
0.15% to 0.3% carbon | Mild steel (low carbon steel) |
0.3% to... | |