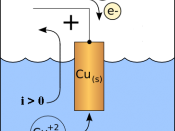
It is known that by passing a constant electric current through an aqueous copper sulphate solution that the passage of ions through this solution results in copper atoms being dissolved into the solution from the anode while positive copper ions (cations) being discharged at the cathode. Normally anions are discharged at the anode.
The experiment carried out aimed to monitor the quantity of Copper (Cu) metal deposited during the electrolysis of Copper Sulphate solution (CuSo4) using Copper electrodes, when certain variables were changed. It was considered that the following factors could affect the deposition of Copper metal on the cathode.
1. Time
2. Current
3. Temperature
4. Molarity/Concentration of Solution
5. Quantity of Solution
6. Size of Electrodes
7. Distance between the electrodes
8. The surface of the electrodes
The time was chosen because it is an easy quantity to measure and record, whilst at the same time maintaining the other variables at a constant level.
The other factors could be observed in later experiments, should time allow.
PREDICTIONS
It is possible to predict that the relationship will be directly proportional between the time the current flows and the mass of Copper deposited on the Cathode (negative electrode). I can therefore predict that if I double the time of the experiment, I will therefore be doubling the charge. This statement can be supported by both of Faraday's Laws.
Faraday's First Law of electrolysis states that:
"The mass of any element deposited during electrolysis is directly proportional to the number of coulombs of electricity passed"
Faraday's Second Law of electrolysis states that:
"The mass of an element deposited by one Faraday of electricity is equal to the atomic mass in grams of the element divided by the number of electrons required to discharge one ion of the element."
Another piece of...