Have you ever wondered what makes that pleasantly tangy taste in your Coca-Cola? How about one of the chemicals that is used in your fertilizer back home? Well that chemical happens to be Phosphoric acid, which is used in several household products. Phosphoric acid might have been in that bar of soap you used this morning to take a shower. In this paper, I will describe where Phosphoric acid is used, its formula, how it is made, who it was discovered by, where it is used, how it is an intermediate product, how it has helped or harmed society, and how it is hazardous.
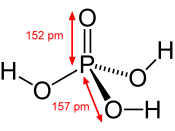
My chemical is called Phosphoric acid, or H3PO4. The commercial method of the preparation is sulfuric acid added to the phosphate rock.
3 H2SO4(l) + Ca3(PO4)2(s) + 6 H2O(l) 2 H3PO4(s) + 3 CaSO4*2H2O(s)
Phosphoric acid is a clear, colorless, syrupy liquid, with a slight acid odor.
Its boiling point is 158úC (316úF), and its melting point 21úC (70úF). Its vapor density is 3.4. Its specific gravity is 1.685.
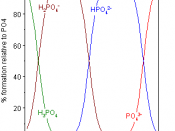
Another name for phosphoric acid is called orthophosphoric acid. Two molecules of it are formed by adding three molecules of water, to one molecule of phosphorus pentoxide, which makes rhombic crystals. The crystals are then melted at about 42úC. It then forms orthophosphate salts with 1 to 3 hydrogens that is replaced by another positive ion. When this is heated to about 225úC, it is formed into phosphoric acid.
Phosphoric acid was discovered by K. W. Scheele and J. G Ghan when they isolated phosphous from bone ash and produced phosphoric acid by the nitric acid on the phosphorus in 1770.
There is around 10 million tons of phosphoric acid produced in the Untied States each year. About 80% of the acid is used in...