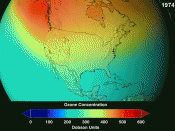
There are several techniques used to investigate the chemistry of thestratosphere. The first of these is monitoring which involvesanalysing the air using spectroscopy. Given that ozone absorbs in theinfra-red and ultra-violet regions of the spectrum, the concentrationon ozone in a sample can be calculated form the strength of itsabsorption (figure1). This has to be carried out at different timesand in different conditions to ensure any decrease is not due tonatural fluctuations.
[IMAGE]Figure 1 - Ozone Distribution in the AtmosphereOnce the molecules present in the stratosphere are identified,laboratory measurements can be carried out to investigate thereactivity of the molecules concerned and how radiation affects them.
Special techniques such as flash photolysis have to be used to workout how fast the reactions are occurring. This technique allowedscientists to work out that the reactions breaking down and makingozone are generally occurring at the same rate and consequently thereis a steady concentration of ozone.
The study of meteorology involveslearning about the movements of air currents which circle around thelines of latitude and help gas to mix within a layer. Meteorologyallows scientists to obtain a better idea of how the reactions occurin the stratosphere as opposed to the very different conditions underwhich they take place in a laboratory. Information from thesedifferent sources is fed into a powerful computer that produces aÃÂmodelÃÂ of what scientists think happens in the stratosphere. The moredata that becomes available the closer to reality the model becomes.
These overall simulations can be used to make predictions about futurevariations in the atmosphere.
[IMAGE]In 1972, James Lovelock, who was interested in the global spread ofgasses in the troposphere, developed a method for detecting CFCs inthe troposphere. He detected small concentrations of CFC 11 in ruralareas, far away from potential sources. He recognised that such astable gas would accumulate and move in the atmosphere.