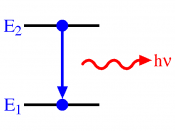
Electric and magnetic components to every light wave-speed of light is always constant-speed of light = wavelength x frequency (c = ûv)Particle Nature of Light-matter can gain or lose energy only in small, specific amounts called quanta-a quantum is the minimum amount of energy that can be gained or lost by an atom-Equantum = hv-h is PlanckÃÂs constant and v is frequency-energy and wavelength are inversely related-for a given frequency matter can emit or absorb energy only in whole-number multiples of h-canÃÂt use the wave description of light to explain this phenomenon (light energy is not wave like, but particle-like of specific sizes)-Photoelectric effect: when light is shone on a metal, electrons may be emitted by the metal-electrons absorb light energy which provides enough energy for theelectrons to leave the atom-the faster the electrons are moving, the more energy was transferredfrom the light-lower frequency (v) number of waves that pass through a point in space per second, s-1, 1/s, Hz --> longer wavelength (û) meters, centimeters â lower energy-shorter wavelength --> higher frequency-if no electrons ejected --> minimum threshold energy required to eject an electron from the metal (light did not provide sufficient energy)-beam of light is a stream of tiny particles called photons-a photon is a particle of electromagnetic radiation with no mass that carries a quantum of energy-Ephoton = hv àh is still PlanckÃÂs constant and v is frequencyAtomic Emission Spectra-electrons absorb a photon (from light) or quantum of energy (from electricity), causing them to move further from the nucleus-to pull the electron away from the positive negativeattraction between the negatively charged electron and the positively charged nucleus --> needs energy-only very specific distances (electrons move farther away fromthe nucleus with quantized amounts of energy in multiples of h àPlanckÃÂs constant which represents a quantum)-this excited...
Reviews of: "Chemistry Study Notes - Electrons and Light"
:
Bibliography:
Chemistry: Matter and Change
by: Dingrando
dreamadream - I'm the writer of these notes and of the previous comment (in which i added the bibliography i forgot)
More Chemistry
essays:
John Dalton: the father of modern chemistry.
... atoms and graphite atoms would be very different. As previously stated, Daltons measurements were not exact. This may have been due to the way he performed his experiments. Dalton preferred to use old and inaccurate tools. This was particularly odd because newer models ...
Determination of Copper by Complexation, Solvent–Extraction and Spectrophotometry
... beam of light through a sample and measuring the intensity of light reaching the detector. The beam of light ... genetics and strategies for modeling dose-response relationships J. Toxicol. Environ. Health B ... ] Although some literature states that the ... higher levels of copper in a daily dose could be higher ...
The Life of John Dalton.
... tiny, indestructible particles called atoms that are all alike and have the same atomic weight. Dalton's studies and writings, many included in his New System of Chemical Philosophy (part I, 1808; part II, 1810), cast light ...
In October 1859, German physicist Gustav Kirchhoff announced the results of his investigations with chemist Robert Bunsen on the dark lines that interrupt the otherwise continuous solar spectrum.
... acceptable research. They bring to light his instrumental and methodological ingenuity, his ... of spectrum analysis.... What really excited De La Rue, a stationer ... capacity to resolve questions about the nature of nebulae. It was ... and 251-252 (1827); 8, 7-10 (1828). H. E. Roscoe to G. G. Stokes, 24 ...

Good
Like the previous comment said, needs a bibliography
0 out of 1 people found this comment useful.