An impure substance is one substance contaminated with small amounts of one or more other substances.
An element is a pure substance which cannot be decomposed into simpler substances.
A compound is a pure substance which can be decomposed into simpler substances, for example into elements.
Pure substance = One single chemical. One single formula. One set of physical properties (behaviours).
Mixture = two or more pure substances. Many formulas. Different physical properties, several of each depending on how the mixture is made.
Iron + sulfurPart is magnetic, part is notPart melts a low temp, part at high temp.
Suspension = two (or more) substances that mix, but will not stay mixed. Usually can be separated by visual inspection. One "settles out." Non-homogeneous (heterogeneous). Separate with a filter or other physical means.
Colloid = two (or more) substances that mix thoroughly. They will stay mixed, but one substance remains in microscopic "clumps" or "droplets;" is does not separate into individual molecules.
Non-homogeneous.
Includes: foam, gel, smoke, aerosolSolution = A homogeneous, complete mixture of two (or more) substances at the molecular level. Molecules of one substance totally separate from each other and mix evenly with molecules of the other substance.
The atmosphere is a mixture of gases. It is predominantly a mixture of the elements nitrogen, oxygen and argon. The hydrosphere consists of different mixtures but mainly water. This includes rivers, lakes and oceans. The lithosphere includes rocks, sand, soils, mineral ores, coal, oil and natural gas. The biosphere is the layer of the earthÃÂs crust in which life exists.
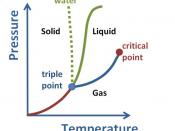
The melting point of a solid is the lowest temperature at which the solid changes to a liquid.
If the melting point is sharp and if it does not increase after submitting the solid to a further purification process, then we...