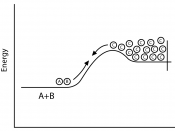
GlossaryItemCategoryDefinitionActivation EnergyReaction rateThe minimum amount of energy required for a reaction to occur.
CatalystReaction rateA substance that speeds up a chemical reaction without being consumed by the reaction.
Collision TheoryReaction rateDescribes the rate of a chemical reaction through different types of collisions of particles.
DissociationThe decomposition or break down of complex molecules into simpler atoms or particles.
EquilibriumEquilibriumEstablished when both forward and reverse reactions have equal reaction rates.
Equilibrium constantEquilibriumThe ratio of product concentrations to reactant concentrations.
ExothermicReactionsA reaction where reactants release energy to form new products.
Le Chatelier's PrincipleEquilibriumDetermines the position of equilibrium of a reaction.
Position of equilibriumEquilibriumShows if the products or reactants produce more.
Reaction rateReaction rate / collision theoryThe change in concentration of a reactant or product in a certain time.
AbstractThe article written by, Jayant M Modak, focuses on the production of ammonia through the Haber process, including a thorough explanation of the chemistry behind this process and background information of ammonia production.
Ammonia is used all over the globe as chemical fertilizers, explosives and as pharmaceutical and household cleaning products [1]. However, what all these have in common is that they all require ammonia for their production. Due to the large demand for ammonia the Haber process was formed to produce the worlds second most produced chemical, ammonia. The equilibrium reaction of ammonia synthesis, requires the abundant nitrogen and hydrogen in the air, iron as a catalyst and 250-400 degrees Celsius of heat for the reaction to occur. This exothermic reaction releases 45.7 KJ/mol of energy at 298K or 25ðc which is considerably high [3].
By using Le Chatelier's principle's and the collision theories, is known that the reaction for the synthesis of ammonia is most effective at low temperatures, high pressures and by using increased concentrations of reactants, which all shift...
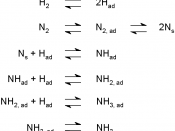
Understanding
For a better understanding,
please download the attachment which shows the appendix items, graphs and tables.
0 out of 0 people found this comment useful.