Introduction
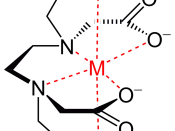
EDTA (HâÂÂY) is widely used to determine metals in complexometric (a volumetric analysis where the formation of a coloured complex is used to indicate the end point of a titration) titrations as it forms stable complexes with most metal ions. EDTA is a tetracorboxylic acid and in alkaline conditions, it exists as Yâ´â» ions, which form 1:1 complexes with metal ions like nickel(II) ions:
Yâ´â» + Niò⺠NiYòâ»
A metal ion indicator (an organic dye which changes colour when it binds with metal ions) shows the end of an EDTA complexometric titration. However, for a metal ion indicator to be suitable in n EDTA titration, it must not bind as strongly with metal ions as EDTA does. Murexide is therefore suitable.
Aim
The aim of this experiment is to determine the percentage of nickel in a nickel(II) salt using EDTA.
Method
The following apparatus was collected:
50cmᶠburette âÂÂGlass stirring rod
20cmᶠpipette âÂÂHydrated nickel(II) sulphate (NiSOâÂÂ.6HâÂÂO)
100cmᶠstandard flask âÂÂStandardised 0.10mollâ»ù EDTA solution
250cmᶠconical flask âÂÂ1 mollâ»ù ammonium chloride
Weighing bottle âÂÂMurexide indicator
Balance (accurate to 0.01g) âÂÂ0.88 aqueous ammonia
100cmᶠbeakers âÂÂDeionised water
25cmᶠmeasuring cylinder
Wash bottle
Pipette filler
White tile
Filter funnel
Approximately 2.6g of hydrated nickel(II) sulphate was transferred to a weighing bottle and the contents weighed and about 25cmᶠof deionised water was added to a 100cmᶠbeaker and the nickel transferred to the water. The bottle was weighed without any remaining salt and then the mixture was stirred until the solid was dissolved before the resulting solution was transferred to a 100cmᶠstandard flask.
The beaker was then rinsed several times with deionised water and the rinsings were added to the standard flask. The solution was filled up to the graduation mark with deionised water and the flask was stoppered and...