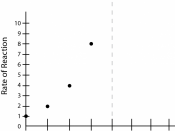
Steven Alderdice Burnage Boy aldy@nme.com Biology Coursework This experiment will find out how temperature effects the rate of reaction between yeast and glucose.
The reaction is as follows C6 H12 O6 yeast 2C2H5OH + 2CO2 Glucose Ethanol + Carbon Dioxide For the reaction to take place particles within reacting substances must collide. The rate of reaction depends upon how often and how hard particles collide with each other. In an enzyme controlled reaction the number of collisions is influenced by the concentrations of the enzyme and its substrate. Enzymes are biological catalysts and they speed up the rate of reaction. This is achieved by the fact that collisions require less energy in the presence of a catalyst to be successful. Therefore more collisions are successful and hence the reaction is speeded up The factors that can influence this rate of reaction are: "â The temperature; An increase in temperature produces an increase in the rate of reaction.
When a solution is heated the particles move faster. As the particles move faster they travel further and are therefore involved in more collisions. However, enzymes work well within a very narrow range and above temperatures of 50ÃÂú C the shapes of enzymes are changed and they become denatured and can no longer combine with substances.
"â Enzymes; An increase in the amount of yeast cells leads to an increase of zymase enzymes and this means an increase in the amount of molecules produced by the enzymes. The more enzyme molecules there are the more successful collisions between particles and so the faster the rate of reaction. Enzymes work as biological catalysts, reducing the amount of energy required for successful collisions.
"â The concentration of glucose and yeast in water. The more glucose and yeast the greater will be the rate of reaction...