Abstract
This study was undertaken to determine the effects of different environments affecting the rate of reaction, PNPP (p-nitrophenyl phosphate) + H20 ? PNP (p-nitrophenol) + H3P04. This reaction is catalyzed by the enzyme phosphatase. Different environments produced different reaction rates as environmental factors affect the efficiency of phosphatase. This is because environmental factors can change the tertiary structure of phosphatase, which alters its active site, and thus changes its efficiency to catalyze the reaction. We measured the rate of reaction, by using a chromogenic substrate, PNPP, and a spectrometer to determine the amount of product produced in 15 min and also using our normal curve created of known PNP concentrations and absorbencies. The optimal conditions for phosphatase which produced the highest rate of reactions were pH of 5 and a temperature of 50úC. We also discovered that increasing the concentration of phosphatase will increase the rate of reaction regardless at what our initial concentration of phosphatase is.
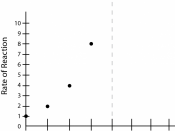
However, by increasing PNPP, the substrate, there are significant increases in the rate of reaction when the initial concentrations of PNPP are low, but when concentrations of PNPP are already high, increases in PNPP concentration resulted in little or no increase in the rate of production of PNP.
Introduction
An enzyme is a protein that catalyzes a chemical reaction. An enzyme works by increasing the rate of reaction by bringing reactants together in the proper orientation and thus decreasing the activation energy. Activation energies drop because enzymes destabilize bonds in the reactant, stabilize the transition state, make acid-base reactions more favorable, and change the reaction mechanism through a covalent bonding interaction (Freeman 2002). Enzymes also catalyze specific reactions because its active site is able to accept one or a very limited number of substrate molecules that will be able to bind to...