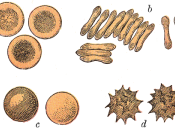
Effects of Solutes with Varying Tonicities on Sheep Red Blood Cells
Introduction
The objective of this lab is to evaluate the effects of solutions with varying tonicities on the sheep red blood cell (SRBC) by examining the response of the cell to varying osmotic conditions (specifically the effect of hypertonic, hypotonic and isotonic solutes on cell membranes.) Henri Dutrochet, (Nov. 1776 - Feb. 1847), a French physiologist, discovered and named the phenomenon of osmosis. [1] Osmosis is the movement of solvent molecules through a selectively permeable membrane into a region of higher solute concentration, with the goal of equalizing the solute concentrations on both sides of the cell. The difference between osmosis and diffusion is that diffusion is the process where molecules move from an area of high concentration to an area of low concentration while in osmosis the molecules move across a selectively permeable membrane. Diffusion and Osmosis are both types of passive transport.
This is because no energy is needed for the molecules to move in or out of the cells. Because biological membranes are semipermeable to larger and/or polar molecules including, but not limited to, proteins, polysaccharides, ions and water, osmosis is needed to facilitate many chemical reactions throughout the body (cells).
Osmotic pressure is the force that moves water through the membrane. A Hypertonic solution has more solute which means there is less water than in the cell. A cell placed in this solution lose water causing the cell to become crenated. A Hypotonic solution has less solute (so MORE water) than the cell. A cell placed in this solution will take up water (osmosis) and blow up. An Isotonic solution has just the right amount of solute for the cell. A cell placed in this solution will stay the same. [2] How will the SBRC...