Electrolysis in chemistry is the movement of an electric current through an ionic substance that is either molten or dissolved. The result of electrolysis is chemical reactions at the electrodes and separation of material as well.
Electrolysis cannot be completed without the following components:
An electrolyte solution: which is defined as a substance that has free ions, which are able to carry the electric current given within the electrolyte solution. It is important that the ions can move, because if the ions are unable to move electrolysis cannot happen because the electric current cannot pass through solid salts.
A D.C current, also known as direct current: this is current that supplies the electrolysis process. This energy supplied by the current is used to create or discharge the ions found in the electrolyte." Electric current is carried by electrons in the external circuit." (1)
Two electrodes: these electrodes are made up of conductive material usually metal, graphite or semiconductors and the choice of the electrode depends on the reactivity it shares with the electrolyte.
These electrodes act like an electric conductor, which enables physical interference between the electrical circuit that is providing the energy and the electrolyte as well.
Process of electrolysis
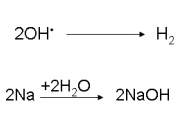
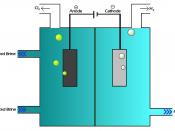
The most crucial process of electrolysis is the exchange of ions and atoms by removing or adding electrons from the external circuit. When the electrolysis process is done with, the results are usually in a different physical state from the molten or dissolved electrolyte solution, either gas or solid form. An example of this physical change is the electrolysis of the element brine. When electrolysis is undergone two gases are formed, hydrogen and chlorine. These products bubble from the electrolyte solution, they are later collected. The reaction in the electrolysis process can be seen in this half redox equation.