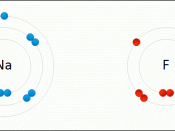
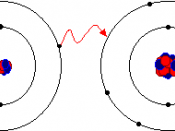
A chemical bond is the energy that holds two atoms together in a compound. This energy can be further broken down into ionic bonding and covalent bonding. An ionic bond is a chemical bond characterized by attraction between ions of opposite charge. The formation of an ionic bond involves a complete transfer of electrons between atoms, and can be predicted when one atom has a much higher electronegativity than the other. A covalent bond is a very strong chemical bond formed by the sharing of electrons. Multiple covalent bonds can be formed when multiple pairs of electrons are shared between atoms. Covalent bonds are generally characterized in two types, polar and non-polar covalent bonds.
Electronegativity is a relative measure of the attraction that atoms of an element have for electrons. The higher the electronegativity of an atom, the stronger its attraction will be for electrons. The type of bond formed between two atoms (ionic or covalent) can be predicted by the difference in electronegativities of the two bonding atoms.
Molecular Geometry is the overall arrangement of the atoms in a molecule. The bonded atoms in a molecule are responsible for determining the molecular geometry of a system. Non-bonded electron pairs are not considered in determining molecular geometry. The molecular geometry consists of six different shapes. Linear, trigonal planar, tetrahedral, trigonal pyramidal, trigonal bipyramidal, and octahedral.
Melting point, boiling point, and vapor pressure are related to the strength of attractive forces between molecules. These attractive forces are called Intermolecular Forces. There are three types of intermolecular forces: ionic, dipole-dipole, and hydrogen bonding. In ionic bonding opposite charges attract each other, ionic forces also hold many ions in a crystal lattice structure. Polar covalent molecules are sometimes described as "dipoles", meaning that the molecule has two "poles". One end of the...