Chrysilla Angelia 10A
Chemistry - Energy Changes in Electrolysis
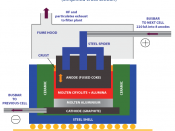
Electrolysis is the use of electric current to stimulate a non-spontaneous reaction. Electrolysis can be used to separate a substance into its original components (elements) and it was through this process that a number of elements have been discovered and are still produced in today's industry. Electrolysis is a process by which electric current is passed through a substance to affect a chemical change. The chemical change is one in which the substance loses or gains an electron (oxidation or reduction).
Electrolysis involves the decomposition of a molten or aqueous compound by electricity. The process is carried out in an electrolytic cell, an apparatus consisting of positive and negative electro d es held apart and dipped into a solution containing positively and negatively charged ions. The compound decomposed during electrolysis is called an electrolyte. An electric current provides the energy that causes the chemical change during electrolysis.
An electric current is simply a flow of electrons. In Electrolysis, an electric current it sent through an electrolyte and into solution in order to stimulate the flow of ions necessary to run an otherwise non-spontaneous reaction. Processes involving electrolysis include: electroplating, electro-refining, electro-synthesis etc.
An important use of electrolytic cells is in the electroplating of silver, gold, chromium, and nickel. Electroplating is the process of depositing a layer of any desired metal on another material by means of electricity. It is one of the most common applications of chemical effects of electric current. The metal to be electroplated is made the cathode, while the anode is the other metal that has to be deposited on this metal. After passing electric current through the electrolyte, the metal from the anode is deposited on the cathode.
Electroplating is basically the process...