8.5.3
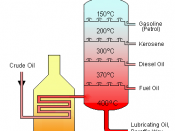
v Fractional distillation of petroleum is performed to obtain products which are useful for use as solvents, polymer products, road-making materials, lubricants and pharmaceuticals. Products from fractional distillation of petroleum are petrol, kerosene and diesel. The separation of petroleum into its 'fractions' depends on differences in the boiling points of the mixture's various components. If the components have similar boiling points, the separation cannot be completed, resulting in the distillate being only slightly richer in the more volatile (lower boiling point) component. If distillations of mixtures with similar volatilities are repeated, improved separations of the components can be achieved.
The steps of fractional distillation are as follows:
1. The mixture of liquids is heated up to 600ÃÂC
2. The mixture boils, forming gases; most substances in mixture become vapour
3. The vapour enters the bottom of fractional distillation column which has layers of trays with bubble caps to allow passing of vapour.
4. The vapour rises through the column
5. The column is cooler at the top than the bottom so the vapour cools as it rises
6. When the vapour reaches a height where the temperature of the column is cooler than its boiling point, it condenses into a liquid.
7. Different liquid fractions are collected by the trays, which can be cooled further for storage or can be processed further.
v When molecular compounds of hydrocarbons have the same molecular formulae but have differing arrangements of atoms, they are called isomers. To distinguish between isomers the IUPAC (International Union of Pure and Applied Chemists) developed a systematic naming procedure. The simplified IUPAC rules for naming straight chain hydrocarbons are:
1. Identify and name (using appropriate prefixes and suffixes) the longest unbranched chain which contains the double or triple bond. If there are no double or triple bonds...