Mobility of Transition Metals as an Affect of Environmental Acidity
Written by: Lene Bruheim
Introduction
Many industries rely on the use of metal processing to produce items for consumers. Metals escape into the biosphere unintentionally, and may disrupt the intended biological functions of biomolecules in a potentially negative manner. Metal pollutants are non-biodegradable, leading their accumulation in a certain region over a period of time. It is important to study the mobility of these metals in order to predict and account for any adverse reactions their accumulation may cause. Chromatography paper was used to measure the Rf value of several transition metals, to better understand the mobility of those transition metals.
Materials and Methods
This laboratory consisted of two experiments. The first one called for the students to identify transition metal cations and the second, to identify their mobility. For experiment one, the dropper from the cation bottles was used to place 3 drops of Fe3+, Co2+, Ni2+, Cu2+, and Zn2+ onto the spot plate in different wells for each solution.
Their color was noted, and then 3 drops of HCl were added from a dropper bottle as well. The changes in color noted, and then the plate cleaned. Again, 3 drops of each solution was placed into separate wells, but this time 3 drops of NH4OH was added and the color change of each was noted. Then the plate was cleaned again, the solutions reapplied in their appropriate wells in 3 drop increments, and the final solution of dimethylglyoxime was added in 3 drop increments as well. Then the color change was noted and cations identified through the use of a chart in the book.
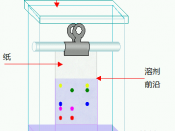
For experiment two, the use of two pieces of chromatography paper was needed. On this paper, a pencil was used to draw a line...