�PAGE �
Title: Experiment on Quantitative Determination of Ksp
Date the Experiment was performed:
Name:
Partner's Name:
Pre-lab: Determination of Ksp
Experimental purpose
The solubility of chemical substances in water often varies greatly depending on their chemical structure and the prevailing temperatures. Due to these differences, the level of solubility can range between being very soluble and insoluble. The solubility of the presumably insoluble substances can be expressed in terms of a constant referred to as Solubility Product Constant (Ksp). In this experiment, Le Chatelier's Principle will be reinforced through exposure of solubility equilibria to chemical perturbations.
Aims and Objectives of study
To establish dissolution constant for precipitates upon addition of chemical reagents
Demonstrate application of LeChaletier's Principle in calculating the concentration of all species at equilibrium.
Laboratory concepts
According to Le Chatelier's Principle (1888): If a system at equilibrium is disturbed by a change in temperature, pressure, or the concentration of one of the components, the system will shift its equilibrium position so as to counteract the effect of the disturbance.
Similarly, the common ion effect concept spells out that addition of an ion from 2 sources pushes the reaction of interest towards the second change from the initial starting conditions and via Le Chatelier's principle, shifts the reaction.
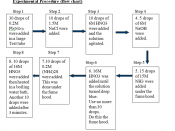
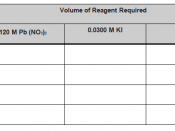
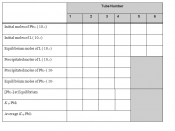
Solubility equilibrium Ksp or Solubility Product, common in most precipitation reactions rely on concepts that can mathematically be expressed in equation as follows:
PbF2(s) ((( Pb2+ (aq) + 2F- (aq)
Ksp = [Pb2+] [F-]2
In this experiment, factors affecting solubility of sparingly soluble salts will be investigated. The experiment is important because salt dissolution helps in increasing ion concentration in solutions, thus affecting the chemical properties of the same solution. Similarly, solubility of salts is affected by temperature and other chemical reagents such as bases, acids, and complexing agents which...