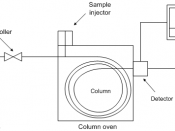
Gas Chromatography
Gas Chromatography is simply the separating of volatile organic compounds. It involves a sample being vaporized and injected onto the head of the chromatographic column. Gas chromatography is used to do many things in today's scientific life. It is used in drunk-driving breath tests and is also used in removing important evidence from crime scenes. Here is a model of a simple gas chromatograph.
Chromatography in general (meaning gas, liquid and any other type of chromatography) separates chemicals using surface interactions. For instance, different chemicals have different tendencies to move through small spaces stick to surfaces. This can be observed in everyday life when you put a drop of water and a drop of glue on an inclined plane. The water will roll off, but the glue will stick. Single molecules can also stick very differently to surfaces. For example, water will condense on a cold glass, but just the single oxygen atom in the air will not.
This is because the bonds with glass that oxygen can make are too weak to hold the oxygen there.
In gas chromatography molecules are forced to pass through a porous medium, which acts as the sticky surface in the previous example. A porous medium is a material that has microscopic holes in it. The holes let molecules pass from one side of the medium to the other.
This is a shot of a chromatograph recorder. The blue area is the porous medium. The molecules start at the top and are collected at the bottom. The red and green particles represent two different molecules. In gas chromatography, these are typically carried by an inert gas. The inert gas is forced through the medium with pressure. However, gas chromatography can also be used to separate gases without a carrier. Molecules...