During the World War I, Germany was dependent on sodium nitrate deposits in Chile for the nitrogen-containing compounds needed to make explosives. Seeking to free Germany from dependence on Chile, a German chemist named Fritz Haber examined the direct combination reaction between nitrogen and hydrogen, a reaction that forms ammonia.
If we decrease the concentration of ammonia in the system, this decreased concentration of ammonia is a stress externally applied to the above system. According to LeChatelier's Principle the system will favor the process that replaces the ammonia that we siphoned off. The forward reaction or process produces ammonia. On other hand, if we increase the concentration of Nitrogen that would be a stress. The system will have to respond in order to undo the stress of increased Hydrogen. The forward process uses up Hydrogen and Nitrogen while the reverse process increases the presence of Nitrogen and Hydrogen.
The industrial conditions for producing ammonia the temperature must be 450úC to 500úC.
The forward reaction is exothermic. If we remove heat as a product will result in the equilibrium mixture making richer ammonia. Since we want ammonia from the Haber process, the reaction conducted at 450úC. Because all reactions go faster if the temperature is raised. Reversible reactions, such as the Haber process, rasing the temperature will make the equilibrium mixture richer in nitrogen and hydrogen because forming these from ammonia takes heat in. If we cool the reaction down the proportion of ammonia will increase but the rate of production will decrease. With a reversible reaction, a catalyst which increases the rate will increase the rate of both the forward and the backward reaction. This is useful because the catalyst will, cause the reaction mixture to reach its equilibrium composition more quickly. The catalyst will not change the...
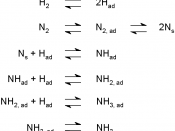

Pretty good
This was a pretty good report/essay. it was a bit too short but other than that it was very nicely structured and sounded good and flowy.
0 out of 0 people found this comment useful.