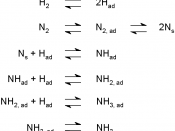THE HABER PROCESS
"ïIn the late 19th century, more food was required to feed Europe and North America's growing population.
"ïThe addition of nitrogen based fertilizer (NaNO3(s), NH3NO3(s)) increased crop yield.
"ïSoon, there was a high demand for the fertilizer itself.
"ïIn 1909, the German company BASF investigated the possibility of producing ammonia from atmospheric nitrogen, N2(g). An year later, Haber, a professor did it.
"ïThe observations were: - N2(g) and H2(g) form an equilibrium mixture with ammonia
- Optimum conditions: closed container, suitable catalyst (iron oxide), a 600 âXC temperature, a 30 MPa pressure
"ïThis method is called the Haber Process
"ïBASF brought the rights to the process and built a plant producing 10 000 t ammonia per year
TEMPERATURE
"ïAt low temperatures, the reaction of N2(g) and H2(g) is not economical
"ïAdding heat increases the rate of reaction
"ïThe higher the temperature, the lower the ammonia yield
"ïHaber had to balance the rate of a reaction (increased by increasing temperature) against the equilibrium of the reaction (pushed to the right by decreasing temperatures)
"ïUsing a catalyst eliminates the need for high temperatures allowing the equilibrium to move to the right at low temperatures
"ïToday, we are using the Haber process to produce huge quantities of ammonia
"ïAmmonia is used to make explosives and fertilizers - dissolves in moisture present in soil (if the soil is acidic it's converted into ammonia ion) and enters the nitrogen cycle where it is converted to nitrate ions by soil bacteria.
Nitrate ions are absorbed by plants through roots and used in proteins, chlorophyll and nucleic acids
"ïWithout nitrogen, the plants produce yellow leafs which cause the plant to dies prematurely
UNDERSTANDING CONCEPTS
1. 1. Add N2(g) - this will increase the reactant concentration. The system will therefore try to balance the equilibrium...